The availability of an oral selective estrogen receptor degrader (SERD) would be good news for patients and clinicians as an alternative to intramuscular fulvestrant. The novel agent LSZ102 might fit the bill, based on early activity shown in combination with the targeted agents ribociclib and alpelisib, in a study reported by Komal Jhaveri, MD, FACP, of Memorial Sloan Kettering Cancer Center, New York, at the 2020 ESMO Breast Cancer Virtual Meeting.1
“This is the first clinical report of an oral SERD in combination with a CDK4/6 inhibitor or an alpha-specific PIK3CA inhibitor,” Dr. Jhaveri said. “The drug was safe and relatively well tolerated alone and in combination and showed clinical activity in the dose-escalation cohorts of the combination arms. We also saw downregulation of the estrogen receptor protein across all arms.”
Dr. Jhaveri noted that most patients ultimately develop resistance to endocrine therapy via resistance pathways, including those for cyclin D–CDK4/6/retinoblastoma protein and PI3K/AKT/mTOR. Additional resistance mechanisms involve mutations in ESR1, which drive estrogen receptor–dependent transcription and proliferation in the absence of estrogen.
Fulvestrant, the only currently approved selective estrogen receptor degrader, has demonstrated a survival benefit in combination with the CDK4/6 inhibitor ribociclib and the PIK3CA inhibitor alpelisib. ESR1 mutations appear to predict resistance to aromatase inhibitors but do not seem to influence outcomes in patients treated with fulvestrant.
“However, fulvestrant has its own limitations,” Dr. Jhaveri pointed out. “Oral SERDs offer an attractive alternative to fulvestrant and could potentially achieve higher systemic exposures, leading to enhanced efficacy and potential activity on ESR1 mutations.”
Study Design
The phase I/Ib, open-label, dose-escalation study, presented as a “Best Abstract” at the 2020 ESMO Breast Cancer Virtual Meeting, evaluated LSZ102 in 193 patients with progressive disease after endocrine therapy. It was studied as a single agent and in combination with ribociclib and alpelisib, in a wide range of dosing, in the following noncomparative and sequential arms:
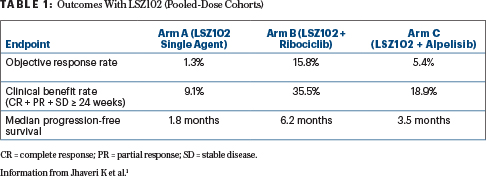
- Arm A (n = 78): LSZ102 as a single agent (prior fulvestrant, CDK4/6 inhibitors, and chemotherapy were allowed)
- Arm B (n = 76): LSZ102 plus ribociclib (prior fulvestrant, CDK4/6 inhibitor, and chemotherapy were allowed)
- Arm C (n = 39): LSZ102 plus alpelisib (prior fulvestrant, CDK4/6 inhibitor, and chemotherapy were allowed, but prior therapy with PI3K, mTOR, or AKT inhibitors was not).
The overall study population was heavily pretreated, having received a median of three prior lines in arm C and four prior lines in arms A and B. More than 50% of patients had prior fulvestrant and 70% had prior chemotherapy in all three arms. Prior CDK4/6 inhibitors were used in 60% of arm A, 70% of arm C, and 40% of arm B. This presentation represents the first public disclosure of results in arm C.
Safety Profile
The most frequent adverse events were gastrointestinal toxicities. Across all arms, nausea (all grades) was observed in 50% to 60% of patients, and diarrhea occurred in 35% of patients in arm B and 60% of patients in arm C. The grade 3 toxicities of neutropenia (13.2%) and elevated levels of aspartate transaminase (3.9%) in arm B were likely driven by ribociclib, whereas in arm C, the grade 3 hyperglycemia (10.0%) and skin rashes (15.4%) appeared to reflect “known alpelisib signals,” noted Dr. Jhaveri.
“The 300-mg currently approved dose of alpelisib was not tolerated well in combination with 300 mg of LSZ102. Five dose-limiting toxicities at this level occurred in 12 patients. The recommended dose was established to be 300 mg of LSZ102 and 250 mg of alpelisib,” she said.
Table 1 shows the outcomes of the three arms treated with LSZ102, with dose cohorts pooled. After data cutoff, one additional partial response was observed in arm C. No dose-dependent activity was observed in any arm, and responses were independent of ESR1 and PIK3CA mutation status, although conclusions for arm C are limited by the small sample size. Estrogen receptor protein levels were measured at baseline and after cycle 1. Downregulation of this protein was persistently observed across the arms, with no substantial dose dependency shown.
Mutation status, by circulating tumor DNA, showed the most common mutations to be ESR1, PIK3CA, and TP53. Clinical activity of the combinations was seen regardless of ESR1 mutational status. The study also found no enrichment of most mutated genes in end-of-treatment samples, regardless of the type of best response.
DISCLOSURE: Dr. Jhaveri has served as a consultant or advisor to Novartis, Pfizer, Genentech, Lilly, AstraZeneca, Bristol-Myers Squibb, ADC Therapeutics, Taiho Oncology, Jounce Therapeutics, and AbbVie and has received traveling reimbursement from Novartis, Pfizer, AstraZeneca, and Jounce.
REFERENCE
1. Jhaveri K, Juric D, Yap Y, et al: Interim results of a phase I/Ib study of LSZ102, an oral selective estrogen receptor degrader, in combination with ribociclib or alpelisib in patients with ER+ breast cancer who had progressed after endocrine therapy. 2020 ESMO Breast Cancer Virtual Meeting. Abstract LBA1. Presented May 24, 2020.

