In the phase II LOTUS trial, the addition of the AKT inhibitor ipatasertib to paclitaxel in the first-line treatment of patients with advanced triple-negative breast cancer yielded a strong numerical improvement—a median gain of 9 months—in overall survival, in the final survival analysis reported by Rebecca Dent, MD, of the National Cancer Centre Singapore, at the 2020 ESMO Breast Cancer Virtual Meeting.1

Median overall survival of more than 2 years represents a meaningful outcome in metastatic triple-negative breast cancer.— Rebecca Dent, MD
Tweet this quote
“Median overall survival of more than 2 years represents a meaningful outcome in metastatic triple-negative breast cancer,” Dr. Dent commented. “In all biomarker-defined subsets—IHC PTEN-normal or -low and PIK3CA/AKT/PTEN-altered or normal—the median overall survival favored ipatasertib plus paclitaxel.”
Importance of PI3K/AKT Pathway
“We have known for some time that the PIK3CA/AKT pathway is important in driving carcinogenesis,” explained Dr. Dent. “We have learned that AKT can be activated by loss of function in negative regulators, such as PTEN; by gain of function in positive regulators, such as PIK3CA and AKT mutations; and by a therapy-induced survival response. When the AKT inhibitor ipatasertib is given with a taxane, the combination is not just additive, but synergistic.”
AKT inhibition is being evaluated in triple-negative breast cancer because of the high prevalence of genetic aberrations in this subtype. In the LOTUS trial, the treatment effect seen with ipatasertib has been most pronounced in patients harboring PIK3CA, AKT, and PTEN alterations, said Dr. Dent.
LOTUS Design
LOTUS was a global, double-blind, placebo-controlled, randomized, phase II trial consisting of 124 patients with previously untreated, locally advanced or metastatic triple-negative breast cancer. Three-fourths of the women had visceral metastases, and some had relapsed as early as 6 months after their last chemotherapy.
Patients were randomly assigned to paclitaxel plus placebo (n = 62) or ipatasertib at 400 mg daily (n = 62). Overall survival in the intent-to-treat population, in patients with low PTEN expression based on immunohistochemistry (IHC), and in patients with PI3K/AKT pathway activation weresecondary endpoints.
The co-primary endpoint was progression-free survival in the intent-to-treat and PTEN-low populations, which was improved with the addition of ipatasertib, as reported in 2017.2 In the intent-to-treat population, the median progression-free survival was 4.9 months with paclitaxel alone vs 6.2 months with the addition of the AKT inhibitor (hazard ratio [HR] = 0.60; 95% confidence interval [CI] = 0.37–0.98). An even greater benefit was shown in the subgroup with PI3K/AKT/PTEN alterations, where the median overall survival was 4.9 months vs 9.0 months, respectively (HR = 0.44; 95% CI = 0.20–0.99).
Final Overall Survival: 9 Months’ Benefit
The final overall survival in the intent-to-treat population had a data cutoff of September 3, 2019. The median follow-up was 16 months in the control arm and 19 months in the experimental arm.
Median overall survival was 16.9 months with paclitaxel alone and 25.8 months with paclitaxel plus ipatasertib (HR = 0.80; 95% CI = 0.50–1.28). The 1-year survival increased by 15%, from 68% to 83%, Dr. Dent reported.
“This is a clinically meaningful improvement of 9 months of median overall survival, which is not too dissimilar to what we see in patients with triple-negative breast cancer and positive expression of PD-L1 [programmed cell death ligand 1] who are receiving checkpoint inhibitors in combination with chemotherapy,” Dr. Dent observed. “The stratified hazard ratio is 0.80, but the confidence interval crosses 1, and therefore we need confirmatory phase III results.”
By IHC PTEN status, patients with both PTEN-normal and PTEN-low tumor showed a numerical improvement in overall survival. However, the sample sizes were too small to draw definitive conclusions, she said (Table 1).
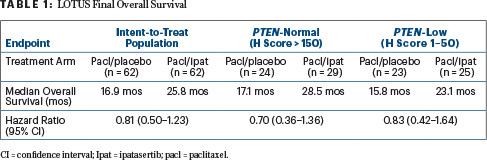
In patients identified as having PIK3CA/AKT/PTEN alternations by next-generation sequencing, there was a “numerical improvement in overall survival and clearly a hint of activity,” which will be further evaluated in a confirmatory phase III trial, Dr. Dent declared, reporting median overall survival times of 22.1 months in the control arm and 25.8 months in the ipatasertib arm (HR = 1.13; 95% CI = 0.52–2.47). For patients without these genetic alterations, median overall survival times were 16.2 months and 23.1 months, respectively (HR = 0.72; 95% CI = 0.39–1.333).
“Given the small sample sizes and the heterogeneity of triple-negative breast cancer, the interpretation of the subgroups is limited,” Dr. Dent cautioned.
KEY POINTS
- The phase II LOTUS trial evaluated the AKT inhibitor ipatasertib plus paclitaxel in 124 patients with advanced or metastatic triple-negative breast cancer.
- In a previous report, the median progression-free survival was significantly improved in both the intent-to-treat population and, more robustly, in patients with tumors with PI3KCA/AKT/PTEN alterations.
- In the final overall survival analysis, the experimental arm had a 9-month improvement in overall survival; this was considered clinically meaningful but did not reach statistical significance, probably because of the small number of patients.
For patients younger than age 50, the risk of disease progression was reduced by 59%, and survival was increased by 20 months with the addition of ipatasertib. Dr. Dent found this “biologically plausible,” possibly reflecting an age group “who may be particularly susceptible to AKT inhibition,” she added.
Subsequent anticancer therapy did not differ between the arms, and no new safety signals were observed. Ipatasertib was associated with more diarrhea and peripheral sensory neuropathy (associated with longer exposure to paclitaxel), but the combination was “extremely well tolerated, especially compared with other agents targeting this pathway,” noted Dr. Dent.
Future Studies
The ongoing phase III confirmatory IPATunity130 trial is evaluating ipatasertib in locally advanced or metastatic triple-negative breast cancer with PI3K/AKT/PTEN-altered tumors. The phase III IPATunity170 trial is evaluating ipatasertib plus paclitaxel and atezolizumab in the first-line treatment of metastatic triple-negative breast cancer.
DISCLOSURE: The LOTUS trial was sponsored by Roche/Genentech. Dr. Dent has received honoraria from Roche, Novartis, Lilly, Pfizer, Eisai, Merck, and AstraZeneca.
REFERENCES
1. Dent R, Antunes De Melo e Oliveira M, Isakoff SJ, et al: Final results of the double-blind placebo-controlled randomized phase II LOTUS trial of first-line ipatasertib plus paclitaxel for inoperable locally advanced/metastatic triple-negative breast cancer. 2020 ESMO Breast Cancer Virtual Meeting. Abstract 139O. Presented May 23, 2020.
2. Kim SB, Dent R, Im SA, et al: Ipatasertib plus paclitaxel vs placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase II trial. Lancet Oncol 18:1360-1372, 2017.


