KEYNOTE-062, a study of first-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma, found pembrolizumab to be noninferior to chemotherapy and perhaps better than chemotherapy in a subgroup of patients. The results were reported at the 2019 ASCO Annual Meeting by Josep Tabernero, MD, PhD, Head of Medical Oncology at the Institute of Oncology at Vall d’Hebron University Hospital, Barcelona.1

Josep Tabernero, MD, PhD
All patients in the study had a programmed cell death protein ligand 1 (PD-L1) combined positive score (CPS) ≥ 1. For this population, pembrolizumab outcomes fell within the noninferiority boundary. The greatest benefit, however, was seen in patients with high expression, ie, CPS ≥ 10. In this group, 2-year overall survival was 39% vs 22% for standard chemotherapy, and median survival was 17.4 months vs 10.8 months. Because of the study’s hierarchical design, this subgroup was not analyzed for statistical significance.
Survival Improvement in PD-L1 High-Expressers
“We saw a clinically meaningful improvement in overall survival with pembrolizumab vs chemotherapy in the PD-L1 CPS ≥ 10 group. Technically speaking, we could not perform an analysis of statistical significance in this group, but the numbers suggest that pembrolizumab is superior,” Dr. Tabernero said.
Similarly, in advanced esophageal carcinoma, KEYNOTE-181 showed that pembrolizumab improved overall survival in patients with a CPS ≥ 10 (hazard ratio [HR] = 0.69; P = .0074), but not in the overall patient population.2
The current guidelines for gastric or gastroesophageal junction cancer are to treat with a platinum plus a fluoropyrimidine in the first-line setting, and with docetaxel, paclitaxel, irinotecan, and ramucirumab, with or without paclitaxel, in the second-line setting. Pembrolizumab is approved for patients with a CPS ≥ 1 after disease progression on at least two lines of chemotherapy.
‘May Well Be Better’ Than Chemotherapy

Richard L. Schilsky, MD, FACP, FSCT, FASCO
At a press briefing, Richard L. Schilsky, MD, FACP, FSCT, FASCO, Senior Vice President and Chief Medical Officer of ASCO, said that in his experience as a gastrointestinal oncologist, gastric or gastroesophageal junction cancer is “a tough disease to treat.” Patients are often frail, elderly, and malnourished—and therefore usually not good candidates for standard cytotoxic chemotherapy.
“This study, demonstrating the potential of immunotherapy to substantially improve outcomes, is important,” he told journalists. “The findings fell within the noninferiority boundary, so it’s reasonable to conclude that pembrolizumab is not inferior to chemotherapy. In addition, it has a substantially improved safety profile. It’s pretty clear to be me that this would be a preferred treatment for this population.”
“Also, though not meeting the definition of statistical significance, it’s quite clear that pembrolizumab is clinically superior to chemotherapy in the high–biomarker-positive population,” Dr. Schilsky continued. “What I take from this study is that for patients with advanced gastric or gastroesophageal junction cancer, pembrolizumab should, in many cases, replace chemotherapy as first-line treatment. It’s certainly not worse and may well be better.”
“We saw a clinically meaningful improvement in overall survival with pembrolizumab vs chemotherapy in the PD-L1 CPS ≥ 10 group.”— Josep Tabernero, MD, PhD
Tweet this quote
KEYNOTE-062 Details
KEYNOTE-062 was a phase III randomized clinical trial of 763 patients with advanced gastric or gastroesophageal junction cancer who were randomly assigned to one of three treatment arms: pembrolizumab at 200 mg every 3 weeks for up to 2 years, pembrolizumab plus chemotherapy (cisplatin and fluorouracil or capecitabine), or placebo plus chemotherapy. All patients had PD-L1 CPS ≥ 1, and 37% had a score ≥ 10.
The study had a complex hierarchic statistical design with four comparisons. The initial hypotheses were tested first, and the remaining hypotheses were tested only after positive results were achieved with the first ones. The initial comparisons and endpoints were:
- Pembrolizumab vs chemotherapy in the CPS ≥ 1 cohort: overall survival, noninferiority
- Pembrolizumab plus chemotherapy vs chemotherapy in the CPS ≥ 10 cohort: overall survival, superiority
- Pembrolizumab plus chemotherapy vs chemotherapy in the CPS ≥ 1 cohort: overall survival, superiority
- Pembrolizumab plus chemotherapy vs chemotherapy in the CPS ≥ 1 cohort: progression-free survival, superiority.
Overall survival differences for pembrolizumab vs chemotherapy in the CPS ≥ 10 cohort could only be determined if superiority was shown for the combination in the CPS ≥ 10 group, and it was not.
Study Outcomes
Compared to chemotherapy, single-agent pembrolizumab was noninferior in the intent-to-treat population and produced a “favorable” effect in the CPS ≥ 10 group (Table 1).
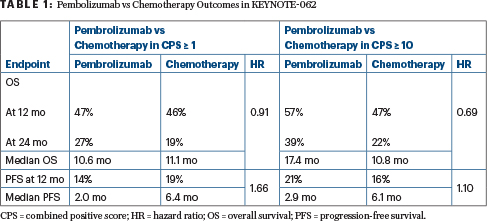
Combining pembrolizumab with chemotherapy did not improve outcomes compared with chemotherapy alone, though the trend was favorable. The hazard ratio was 0.85 both for patients with a CPS ≥ 1 and for those with a CPS ≥ 10. The objective response rate was higher for pembrolizumab plus chemotherapy than with chemotherapy alone, but chemotherapy produced more responses than did single-agent pembrolizumab.
Serious adverse events were lowest among patients receiving pembrolizumab alone. “Patients who received pembrolizumab had fewer adverse events and grade 3 to 5 events, and fewer discontinued therapy due to adverse events,” Dr. Tabernero reported.
Grade ≥ 3 events occurred in 17% of the pembrolizumab group, 71% of the combination group, and 68% of the chemotherapy group. The safety profile of pembrolizumab was consistent with previous reports.
“This is the first presentation of the results, but other ongoing analyses are being done, especially in the field of biomarkers,” Dr. Tabernero added. “Using other biomarkers, perhaps tumor mutational burden, and other CPS cutoffs, we may be able to define other populations who benefit from pembrolizumab.” ■
DISCLOSURE: This study received funding from Merck & Co Inc. Dr. Tabernero is a consultant or advisor for Bayer, Boehringer Ingelheim, Lilly, MSD, Merck Serono, Novartis, Sanofi, Taiho Pharmaceutical, Merrimack, Peptomyc, Rafael Pharmaceuticals, Symphogen, Chugai Pharma, Ipsen, Merus, Pfizer, Seattle Genetics, Array BioPharma, AstraZeneca, BeiGene, Genentech, Genmab, Halozyme, Imugene, Inflection Biosciences, Kura, Menarini, Molecular Partners, Pharmacyclics, ProteoDesign, Roche, Servier, VCN Biosciences, Biocartis, Foundation Medicine, HalioDx, and Roche Diagnostics. Dr. Schilsky has received institutional research funding from AstraZeneca, Bayer, Bristol-Myers Squibb, Genentech/Roche, Lilly, Merck, and Pfizer and has received travel expenses from Varian.
REFERENCES
1. Tabernero J, Van Cutsem E, Bang Y-J, et al: Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction adenocarcinoma: The phase III KEYNOTE-062 study. 2019 ASCO Annual Meeting. Abstract LBA4007. Presented June 2, 2019.
2. Kojima T, Muro K, Francois E, et al: Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: The phase 3 KEYNOTE-181 study. 2019 Gastrointestinal Cancers Symposium. Abstract 2. Presented January 17, 2019.


