A joint analysis of two important phase III clinical trials—TEXT (Tamoxifen and Exemestane Trial) and SOFT (Suppression of Ovarian Function Trial)—showed that exemestane plus ovarian function suppression was superior to tamoxifen plus ovarian suppression in preventing recurrence in premenopausal women with hormone-sensitive breast cancers.
“This joint analysis provides new and long-awaited information on how to treat premenopausal patients with hormone-sensitive breast cancer who have the potential to be cured,” said ASCO Immediate Past President Clifford A. Hudis, MD, FACP, at a press conference during the 2014 ASCO Annual Meeting, where these data were presented at a Plenary Session.1 Study results were published simultaneously online in The New England Journal of Medicine.2
New Adjuvant Option
“Exemestane was previously recommended only for postmenopausal women. This analysis demonstrates that an aromatase inhibitor is effective adjuvant therapy for premenopausal women when combined with ovarian suppression. Exemestane [plus ovarian suppression] is a valid alternative to tamoxifen [plus ovarian suppression] in young women with hormone-sensitive early breast cancer. It is a new adjuvant treatment option that reduces recurrence in this setting,” said lead author Olivia Pagani, MD, Clinical Director of the Breast Unit at the Oncology Institute of Southern Switzerland in Bellinzona. Optimal endocrine therapy for premenopausal women is still uncertain, she said.
Ovarian suppression is used more frequently in Europe than in the United States to achieve low estrogen levels in patients with hormone-sensitive breast cancer. Tamoxifen is considered the standard of care in this setting, and adjuvant chemotherapy is sometimes used depending on the judgment of the patient’s risk by the oncologist and patient preference.
SOFT and TEXT Trials
Both SOFT and TEXT were designed to compared tamoxifen for 5 years plus ovarian suppression vs exemestane for 5 years plus ovarian suppression; SOFT was a three-arm trial comparing both of those treatment approaches vs tamoxifen alone (results of the tamoxifen arm are not yet available). TEXT was a two-arm trial comparing exemestane plus ovarian suppression vs tamoxifen plus ovarian suppression. The joint analysis presented at the ASCO Annual Meeting was based on a total of 4,690 patients from both trials enrolled in the ovarian suppression–containing arms.
Overall, two-thirds of patients were aged 40 to 49; and 42% node-positive; 62.3% had tumors of 2 cm or less; and 12% were HER2-positive. Chemotherapy was given at the discretion of the treating physician, and 42.6% did not receive chemotherapy. In a separate interview, Dr. Pagani explained that 21% of TEXT patients and 8% of SOFT patients who did not receive chemotherapy had tumors larger than 2 cm and some positive nodes, usually considered adverse prognostic features that can lead to the prescription of chemotherapy.
“Exemestane plus [ovarian suppression] could replace chemotherapy at least in some patients. Our study suggests that for some hormone-sensitive tumors, exemestane and [ovarian suppression] can replace chemotherapy,” Dr. Pagani stated.
Key Findings
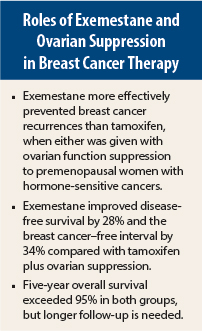
At a median follow-up of 68 months, 5-year disease-free survival was 91.1% with exemestane plus ovarian suppression and 87.3% in the tamoxifen plus ovarian suppression group (P < .001). The 5-year rate of freedom from breast cancer was 92.8% in the exemestane-treated patients compared with 88% in those who received tamoxifen (P < .001).
Among patients who did not receive chemotherapy and were treated with exemestane and ovarian suppression, 97.6% of the TEXT population and 97.5% of the SOFT population were free of breast cancer at 5 years.
Distant recurrence comprised about 60% of the events. The 5-year rate of freedom from recurrence at a distant site was 93.8% in the patients assigned to receive exemestane plus ovarian suppression compared with 92% of those treated with tamoxifen plus ovarian suppression.
The overall survival rates were high in the two groups and not significantly different. At 5 years, overall survival was 95.9% in the exemestane group and 96.9% in the tamoxifen group. However, it is premature to determine survival, Dr. Pagani said.
Side effects of both drugs were similar to what is reported in postmenopausal women. Adverse events that were more frequent with exemestane included fractures, musculoskeletal symptoms, vaginal dryness, decreased libido, and dyspareunia. Those more frequently reported with tamoxifen included thromboembolic events, hot flushes, night sweats, and urinary incontinence. Adherence was very good in both arms, with only 14% of patients interrupting both treatments before 5 years.
Gynecologic cancers were reported in seven exemestane-treated patients and in nine tamoxifen recipients. Endometrial cancers occurred in two and five patients, respectively. About 30% of patients in both arms experienced grade 3 or 4 adverse events.
Convincing Evidence?
At the press briefing, Dr. Hudis said, “This is a long-awaited study that addresses a question rooted in the very beginning of modern breast cancer therapy a century ago. While this result supports the use of [ovarian suppression] and [aromatase inhibitors] in premenopausal women with hormone-sensitive early breast cancer, we still need to see the results of the tamoxifen-alone arm to complete the picture.” ■
Disclosure: Dr. Hudis reported no potential conflicts of interest. Dr. Pagani has received research funding from Ipsen and Pfizer. For full disclosures of the study authors, visit abstracts.asco.org.
References
1. Pagani O, Regan MM, Walley B, et al: Joint analysis of IBCSG TEXT and SOFT trials. ASCO Annual Meeting. Abstract LBA1. Presented Sunday, June 1 2014.
2. Pagani O, Regan MM, Walley B, et al: Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. June 1, 2014 (early release online).