The highly anticipated ENGOT-en9/LEAP-001 trial in endometrial cancer has missed both its primary endpoints. At the Society of Gynecologic Oncology (SGO) 2024 Annual Meeting on Women’s Cancer, the study investigators reported no significant benefit in progression-free survival or overall survival with lenvatinib plus pembrolizumab as first-line treatment of advanced or recurrent endometrial cancer, either in the overall population or in patients with mismatch repair–proficient (pMMR) tumors. Benefit was, however, notable among patients with mismatch repair–deficient (dMMR) tumors.1
Failure to meet the primary endpoints was initially announced by the drug manufacturers in December 2023. At the SGO meeting, Christian Marth, MD, PhD, of Medical University of Innsbruck, Austria, described these and other outcomes. “Lenvatinib/pembrolizumab prolonged progression-free and overall survival vs paclitaxel/carboplatin in subgroups,” he said, referring to the dMMR population and patients who had received prior neoadjuvant or adjuvant chemotherapy, who also had higher response rates and longer durations of response vs the chemotherapy arm.

“Lenvatinib/pembrolizumab prolonged progression-free and overall survival vs paclitaxel/carboplatin in subgroups.”— CHRISTIAN MARTH, MD, PhD
Tweet this quote
“These data confirm that lenvatinib/pembrolizumab is an active combination for endometrial cancer and should be regarded as an important treatment option for advanced disease that is pMMR with disease progression following prior systemic therapy in any setting,” Dr. Marth said.
Dr. Marth noted that in the phase III KEYNOTE-775/Study 309, lenvatinib plus pembrolizumab significantly improved progression-free and overall survival vs physician’s choice of chemotherapy in previously treated patients, both in the overall and pMMR populations.2 Based on these findings, lenvatinib/pembrolizumab became the standard of care for advanced or recurrent endometrial cancer after disease progression on prior neoadjuvant or adjuvant therapy.
The RUBY3 and NRG GY0184 trials showed improved outcomes when checkpoint inhibitors were combined with paclitaxel/carboplatin in the first-line setting. The LEAP-001 study, therefore, compared lenvatinib/pembrolizumab to paclitaxel/carboplatin in the first-line setting.
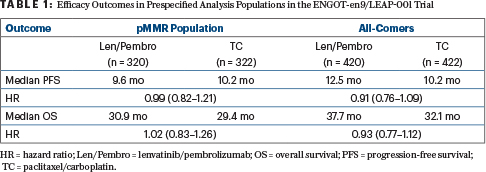
ENGOT-en9/LEAP-001 Details
The phase III LEAP-001 study enrolled 842 patients from 22 countries. Patients were either previously untreated or had shown disease progression at 6 months or longer after neoadjuvant/adjuvant chemotherapy. They were randomly assigned to pembrolizumab every 3 weeks plus lenvatinib at 20 mg daily for up to 35 cycles or paclitaxel/carboplatin for up to 7 cycles. The trial’s dual primary endpoints were progression-free survival and overall survival in the intention-to-treat population and in patients with pMMR tumors.
With a median follow-up of 38.4 months, lenvatinib plus pembrolizumab did not improve overall survival or progression-free survival in the overall or pMMR populations (Table 1). The objective response rates in either arm ranged from 50% to 55% in the primary analysis.
Benefit Seen in Other Subgroups
In contrast to the findings in the prespecified primary analysis populations, findings in other subgroups of patients suggested the combination has benefit. “When we looked at the dMMR subgroup, we observed a prolongation of progression-free survival, increased overall survival, increased overall response rate, and prolongation of response,” Dr. Marth said.
These benefits in the dMMR subgroup of 200 patients included, for lenvatinib plus pembrolizumab vs chemotherapy, the following findings: median progression-free survival was 31.8 months vs 9.0 months (hazard ratio [HR] = 0.61; 95% confidence interval [CI] = 0.40–0.92); fewer deaths were seen in the lenvatinib/pembrolizumab arm, with median overall survival not reached in either arm (HR = 0.57; 95% CI = 0.36–0.91). At 24 months, overall survival rates were 82.9% vs 62.6%; objective response rates were 72.0% vs 58.0%, and median duration of response was not reached, vs 11.7 months.
In patients who had received prior neoadjuvant or adjuvant chemotherapy, findings included the following for lenvatinib/pembrolizumab vs chemotherapy: Median progression-free survival in all-comers was 15.0 months vs 8.3 months (HR = 0.52; 95% CI = 0.33–0.83), and in the pMMR subgroup was 12.5 months vs 8.3 months (HR = 0.60; 95% CI = 0.37–0.97); median overall survival in the pMMR subgroup was 34.2 months vs 21.1 months (HR = 0.67; 95% CI = 0.41–1.11); improved response rates and durations of response were seen with lenvatinib/pembrolizumab.
Quality-of-life analyses showed similar outcomes between the treatments on most subscales. Rates of neuropathy, nausea, anemia, and alopecia were lower with lenvatinib/pembrolizumab, but rates of hypertension, hypothyroidism, diarrhea, and proteinuria were increased.
DISCLOSURE: Dr. Marth reported financial relationships with Eisai and Merck Sharp & Dohme.
REFERENCES
1. Marth C, Moore RG, Bidzinski M, et al: Lenvatinib plus pembrolizumab versus chemotherapy as first-line therapy for advanced or recurrent endometrial cancer: Primary results of the phase 3 ENGOT-en9/LEAP-001 study. Society of Gynecologic Oncology 2024 Annual Meeting on Women’s Cancer. Late-Breaking Abstract. Presented March 18, 2024.
2. Makker V, Colombo N, Herráez AC, et al: Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386:437-448, 2022.
3. Powell MA, Auranen A, Willmott LJ, et al: Overall survival among patients with primary advanced or recurrent endometrial cancer treated with dostarlimab plus chemotherapy in the ENGOT-en6-NSGO/GOG-3031/RUBY Trial. Society of Gynecologic Oncology 2024 Annual Meeting on Women’s Cancer. Late-Breaking Abstract. Presented March 16, 2024.
4. Eskander RN, Sill M, Miller A, et al: Overall survival, progression-free survival by PD-L1 status, and blinded independent central review results with pembrolizumab plus carboplatin/paclitaxel versus placebo plus carboplatin/paclitaxel in patients with endometrial cancer: Results from the NRG GY018 trial. Society of Gynecologic Oncology 2024 Annual Meeting on Women’s Cancer. Late-Breaking Abstract. Presented March 16, 2024.
EXPERT POINT OF VIEW
Robert L. Coleman, MD of Texas Oncology, who is a special advisor to GOG Partners and was the invited discussant for the LEAP-001 trial, commented: “We’ve been anticipating this study for at least a year, so it was really nice to see the data.” Unfortunately, he noted,the study’s two primary endpoints were not met; in hierarchical testing, the noninferiority testing for overall survival in the mismatch repair–proficient (pMMR) population, in particular, resulted in an upper limit hazard ratio just outside that required in the statistical plan. “We could not reject the null hypothesis…. Basically, although the curves look directly overlapping, we would interpret this as inconclusive. But there were some important hypothesis-generating elements,”Dr. Coleman said.

Robert L. Coleman, MD
“In the mismatch repair–deficient (dMMR) population, there is an efficacy signal from the addition of immunotherapy. During chemotherapy, we see absolutely no separation, which is consistent with every single trial that’s been done looking at the addition of immunotherapy,” he said. He noted that the progression-free and overall survival curves began separating around 6 months and that overall survival was improved even with 31% of patients in the control group crossing over to immunotherapy.
“And we also saw, in patients with prior treatment—either neoadjuvant or adjuvant chemotherapy—there seemed to be a difference in the pMMR subgroup,” he said, with a 24-month progression-free survival rate of 26.6% in the immunotherapy arm vs 8.5% with chemotherapy (hazard ratio = 0.60).The suggestion is that patients with prior exposure to chemotherapy seem to be a different subgroup of patients within the pMMR population.
Unanswered Questions
“It begs the question of whether there are potential opportunities that we can take further?” Dr. Coleman said. “It also raises the question, is there an opportunity to focus this [immunotherapy] in the maintenance setting. Can we just give chemotherapy or chemoimmunotherapy and then start to think about what the most appropriate maintenance strategy would be?”
Two trials are underway in dMMR cohorts that are exploring the replacement of chemotherapy with single-agent immunotherapy: GOG-3064/ENGOT en15 KN-C93 (with pembrolizumab) and ENGOT-en13 DOMENICA (with dostarlimab). A future study is also evaluating lenvatinib plus pembrolizumab vs pembrolizumab as maintenance after chemotherapy with and without immunotherapy in pMMR patients.
DISCLOSURE: Dr. Coleman reported financial relationships with Clovis Oncology, Genentech/Roche, AstraZeneca, Genmab/Seagen (Pfizer), Pharma&, GSK, AbbVie, Zentalis, Merck, Alkermes, ImmunoGen (AbbVie), Karyopharm, and Clovis.

