In the global phase III COMMANDS trial of patients with low-risk transfusion-dependent myelodysplastic syndrome (MDS), with or without ring sideroblasts, treatment with luspatercept essentially doubled the likelihood of achieving transfusion independence and an increase in hemoglobin level, compared with epoetin alfa, an erythropoiesis-stimulating agent (ESA), in a planned interim analysis.1
“Luspatercept is the first and only therapy to demonstrate superiority in a head-to-head study against ESAs in transfusion-dependent lower-risk MDS…. This is important, as ESAs have been the first-line treatment for patients with lower-risk MDS for decades,” said Guillermo Garcia-Manero, MD, Professor in the Department of Leukemia and Chief of the Section of Myelodysplastic Syndromes at The University of Texas MD Anderson Cancer Center in Houston.

Guillermo Garcia-Manero, MD
Luspatercept, an erythroid maturation agent, is approved by the U.S. Food and Drug Administration for the treatment of patients with lower-risk MDS who meet certain criteria, including the presence of ring sideroblasts and failure on (or ineligibility for) an ESA, based on the results of the MEDALIST trial.2
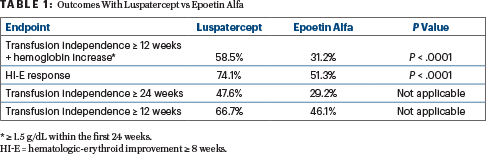
The subsequent global COMMANDS trial evaluated the drug in a wider population that included patients with and without ring sideroblasts. The primary endpoint was transfusion independence for at least 12 weeks within the first 24 weeks with a concurrent mean hemoglobin increase of at least 1.5 g/dL. At the time of this planned interim analysis (representing 80% of participants), 58.5% receiving luspatercept met this endpoint, compared with 31.2% receiving epoetin alfa (P < .0001), Dr. Garcia-Manero reported in a press briefing prior to the 2023 ASCO Annual Meeting.
He maintained that COMMANDS would usher in a “paradigm shift” in the treatment of patients with lower-risk MDS-associated anemia who are naive to prior therapy. “I think that’s going to happen with the presentation of these results…. They will move traditional ESAs over and establish luspatercept as front-line therapy.”
COMMANDS Details
The global phase III COMMANDS clinical trial included 354 patients with lower-risk MDS as defined by the revised International Prognostic Scoring System (IPSS-R) criteria. Patients were ESA-naïve, had less than 5% bone marrow blasts and serum EPO levels less than 500 U/L, and required red blood cell transfusions (defined as 2–6 units/8 weeks for at least 8 weeks immediately prior to randomization).
Patients were randomly assigned to receive subcutaneous luspatercept (starting dose 1.0 mg/kg, titration up to 1.75 mg/kg) once every 3 weeks for at least 24 weeks (n = 178) or epoetin alfa (starting dose 450 IU/kg, titration up to 1,050 IU/kg) once a week for at least 24 weeks (n = 176). At the planned interim analysis of 301 patients, the median treatment duration was 41.6 weeks for luspatercept and 27.0 weeks for epoetin alfa.
Outcomes by Ring Sideroblast Status
“For the primary endpoint, patients receiving luspatercept, regardless of subgroup, achieved transfusion independence with a hemoglobin increase,” Dr. Garcia-Manero said. The drug was effective regardless of baseline serum erythropoietin level, red blood cell transfusion burden, SF3B1 mutation status, or ring sideroblast status.
For patients with ring sideroblasts (n = 220), the primary endpoint was met by 64.8% receiving luspatercept vs 25.9% receiving the ESA. For the ring sideroblast–negative subset (n = 80), this endpoint was met by 41.0% vs 46.3%.
Dr. Garcia-Manero said the disproportionate number of patients with ring sideroblasts was the result of the more common occurrence of this phenotype and the approval of luspatercept in ring sideroblast–positive patients. “The study was not powered to see a significant difference between ESAs and luspatercept in the ring sideroblast–negative context, but the results are not inferior. Actually, they are quite similar,” he said. “Furthermore, look at the duration of response…. Patients receiving luspatercept experienced longer durations of transfusion independence, regardless of ring sideroblast status.”
Median durations of transfusion independence with luspatercept vs epoetin alfa, with hazard ratios (HR) and 95% confidence intervals, follow: all patients, 126.6 vs 77.0 weeks (HR = 0.456 [CI = 0.260–0.798]); ring sideroblast–positive, 120.9 vs 47.0 weeks (HR = 0.626 [CI = 0.361–1.085]); ring sideroblast–negative: not estimable vs 95.1 weeks (HR = 0.492 [CI = 0.148–1.638]).
Secondary Endpoints and Tolerability
The secondary endpoints in the study included hematologic improvement–erythroid response ≥ 8 weeks and red blood cell transfusion independence at 24 weeks and at ≥ 12 weeks. Across these endpoints, as with the primary endpoint, luspatercept was more effective than epoetin alfa (Table 1), Dr. Garcia-Manero reported.
Safety was consistent with previous experience with luspatercept, according to Dr. Garcia-Manero. Adverse events were slightly more common with luspatercept (92.1%) than epoetin alfa (85.2%), as were those considered related to treatment (30.3% vs 17.6%). Treatment discontinuation occurred in 4.5% and 2.3%, respectively.
The most common grade 3 or 4 adverse events attributed to treatment with luspatercept were anemia (7.3%), thrombocytopenia (3.9%), neutropenia (3.9%), and dyspnea (3.9%). Transformation to acute myeloid leukemia was observed in 2.2% of the luspatercept arm and 2.8% of the epoetin arm. All-cause mortality was similar, 18% in each arm.
Dr. Garcia-Manero said the favorable toxicity profile, coupled with the ease of administration (every 3 weeks), “is probably why this drug, in my opinion, will likely become the standard of care for our patients with lower-risk MDS, whether they are ring sideroblast–positive or –negative. I think there will be a paradigm shift for most patients.”
DISCLOSURE: The study was funded by Bristol Myers Squibb. Dr. Garcia-Manero reported financial relationships with AbbVie, Acceleron Pharma, Astex Pharmaceuticals, Bristol Myers Squibb/Celgene, Curis, Genentech, Gilead Sciences, and Novartis.
REFERENCES
1. Garcia-Manero G, Platzbecker U, Santini V, et al: Efficacy and safety results from the COMMANDS trial. 2023 ASCO Annual Meeting. Abstract 7003. Presented at a press briefing May 22, 2023.
2. Fenaux P, Platzbecker U, Mufti GJ, et al: Luspatercept in patients with lower-risk myelodysplastic syndromes. N Engl J Med 382:140-151, 2020.


