Since the introduction of trastuzumab in the late 1990s, overall survival for patients with HER2-positive metastatic breast cancer has substantially improved. Median overall survival in the pivotal first-line trial was only 20.3 months in the chemotherapy arm, and 25.1 months in the trastuzumab/chemotherapy arm.1 In contrast, in the first-line CLEOPATRA trial,2 which enrolled patients between 2008-2010, median overall survival had lengthened to 57.1 months in the taxane/trastuzumab/pertuzumab (THP) arm, with 8-year landmark overall survival of 37%. Further improvements in overall survival were demonstrated in the second-line EMILIA trial.3 Median progression-free survival for ado-trastuzumab emtansine (T-DM1) in the EMILIA trial was 9.6 months and median overall survival was 30.9 months. Due to overlapping enrollment periods, pertuzumab was not routinely given when EMILIA was enrolling. Subsequent “real-world” reports have suggested that the performance of T-DM1 in the post-pertuzumab setting is inferior to that initially reported in EMILIA.4

Nancy U. Lin, MD
Based on the CLEOPATRA5 and EMILIA3 trials, both initially published in 2012, the recommended sequence of therapy over the past decade for patients with HER2-positive metastatic breast cancer has been first-line THP followed by second-line T-DM1.6 Choice of third-line and beyond therapy has historically been flexible, with trastuzumab/chemotherapy or lapatinib-based combinations among the options. More recently, fam-trastuzumab deruxtecan-nxki (T-DXd),7 tucatinib/trastuzumab/capecitabine,8 neratinib/capecitabine,9 and margetuximab/chemotherapy10 have emerged as new options in patients with refractory HER2-positive metastatic breast cancer.
DESTINY-Breast03: Key Findings
As reported in The New England Journal of Medicine—and highlighted in this issue of The ASCO Post—the DESTINY-Breast03 study11 randomly assigned 524 patients with HER2-positive metastatic breast cancer who had been previously treated with a taxane and trastuzumab to T-DXd vs T-DM1. Approximately 60% of patients received prior pertuzumab and approximately 15% received a prior HER2-targeted tyrosine kinase inhibitor.
Striking efficacy was seen with T-DXd. Median progression-free survival by independent radiology review was not reached, with a lower bound of the 95% confidence interval (CI) of 22.1 months, and median investigator-assessed progression-free survival was 25.1 months. Median progression-free survival by independent radiology review in the T-DM1 arm was only 6.8 months (95% CI = 5.6–8.2 months; hazard ratio [HR] = 0.28, 95% CI = 0.22–0.37, P < .001). At 12 months, 75.8% of patients assigned to T-DXd were alive without disease progression, compared to only 34.1% of patients assigned to T-DM1. Though overall survival outcomes are not yet mature, with only 86 deaths across both arms at the time of data cutoff, there is an early signal of potential benefit (12-month overall survival = 94.1% vs 85.9%; HR = 0.55, P = .007), though this did not meet the prespecified threshold for significance.
T-DXd: Toxicity Profile
In considering the clinical utility of T-DXd, the toxicity profile must be carefully assessed, particularly as a benefit in overall survival has not yet been definitively proven. In addition to hematologic toxicity, T-DXd is associated with high rates of nausea and vomiting (72.8% any grade nausea; 6.6% grade > 3; 44% any grade vomiting; 1.1% grade > 3). Hence, patients should receive a two- to three-drug regimen for prophylaxis of nausea and vomiting, according to standard guidelines for moderately emetogenic therapies.12 Another important difference is the potential for alopecia (grade 1 and 2 in 26.5% and 9.5% of patients treated with T-DXd, respectively, vs grade 1 in only 2.3% and grade 2 in no patients treated with T-DM1). How effectively technologies such as scalp cooling prevent hair loss from T-DXd is unknown; an ongoing clinical trial is addressing this question (NCT04986579).
The most feared risk associated with T-DXd is interstitial lung disease.13 Any grade interstitial lung disease was reported in 10.5% of patients treated with T-DXd (0.8% grade 3, no grade 4/5), vs 1.9% of patients treated with T-DM1 (0% grade 3-5). These rates, though still clinically relevant, are reassuring relative to the initial experience with T-DXd and speak to the importance of early recognition and intervention, including permanent discontinuation of T-DXd for grade 2 or higher interstitial lung disease.
Choosing the Optimal Second-Line Regimen
Based on results of DESTINY-Breast03, should T-DXd replace T-DM1 as the preferred second-line regimen in most patients with HER2-positive metastatic breast cancer?
The robust absolute improvements in progression-free survival associated with T-DXd strongly argue in favor of a change to our traditional sequencing paradigm. Indeed, the ESMO Clinical Practice Guidelines have been updated to state that “it is reasonable to consider trastuzumab deruxtecan the new standard second-line therapy in regions where this drug is available.”14 Regarding the counterargument that T-DXd should be “saved” for later-line therapy given high levels of activity in the refractory setting, it is notable that in DESTINY-Breast03, in addition to the substantial progression-free survival benefit, objective responses were also more frequent with T-DXd (79.7% vs 34.2%, P < .0001). Moreover, progressive disease was the best overall response in only 1.1% of patients assigned to T-DXd, versus 17.5% of patients assigned to T-DM1. Certainly, for symptomatic patients, and those with high disease burden and/or rapid disease progression, there is strong rationale favoring T-DXd.
In whom might alternatives to T-DXd be considered? Compared to T-DXd, T-DM1 offers less alopecia, nausea/vomiting, and interstitial lung disease. For patients with low disease burden, a history of interstitial lung disease or other significant pulmonary disease, or frail patients, T-DM1 may still be a reasonable option as part of a shared decision-making process.
What About for Patients With Brain Metastases?
Perhaps one of the most important unknowns with respect to T-DXd is its performance relative to tucatinib-capecitabine-trastuzumab in patients with brain metastases. Patients with brain metastases were only eligible for DESTINY-Breast03 if the metastases were previously treated and stable. Patients with symptomatic brain metastases were excluded. In subset analyses, an overall progression-free survival benefit was observed in patients with stable brain metastases (HR = 0.25, 95% CI = 0.13–0.45) in favor of T-DXd over T-DM1. As reported by Hurvitz et al15 at the 2021 San Antonio Breast Cancer Symposium (SABCS), central nervous system (CNS) objective response rates were also higher (63.9% vs 32.8%); however, the response data include only 36 patients treated with T-DXd, all of whom had previously treated, stable, and asymptomatic brain metastases at baseline, and there was no reporting of time from most recent CNS-directed radiotherapy, which could have contributed to the responses observed. Several small prospective clinical trials and case series have been recently presented that further support high rates of CNS responses with T-DXd, including in patients with active brain metastases, but the total number of patients treated remains small.16-18
In contrast, HER2CLIMB19 enrolled 291 patients with brain metastases, of whom 174 had active brain metastases at baseline (including 66 patients with new, previously untreated brain metastases). Updated analyses presented at SABCS 2021 show an unprecedented 9-month absolute overall survival benefit with the addition of tucatinib to trastuzumab-capecitabine (median = 21.6 vs 12.5 months, HR = 0.60, P = .007).19 Intriguingly, time to CNS progression or death (CNS-progression-free survival) was also substantially improved (HR = 0.386, P < .0001), as was time to new CNS lesions in the overall study population, suggesting a potential CNS prevention benefit. While the evidence is strengthening for potential CNS efficacy of T-DXd, given the strong survival benefits seen with tucatinib-capecitabine-trastuzumab, including in patients with active brain metastases, the evidence base at this time supports selection of tucatinib-capecitabine-trastuzumab over T-DXd in patients with brain metastases, outside of a clinical trial. An exception to this generalization is patients who would have met eligibility for DESTINY-Breast03 (asymptomatic, stable, previously treated brain metastases) in whom extracranial disease progression is the predominant active issue. The ongoing DESTINY-Breast12 trial (NCT04739761) is enrolling up to 250 patients with either active or stable brain metastases and should shed further light on the role of T-DXd in patients with CNS disease.
How Might Sequencing of Anti-HER2 Agents Further Evolve?
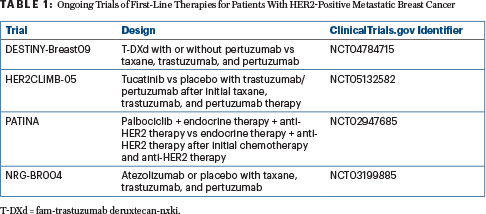
HER2CLIMB-02, which is comparing T-DM1 with or without tucatinib in the second-line setting, has completed accrual and the results are pending. If HER2CLIMB-02 is a positive study, where T-DM1/tucatinib ultimately lands in the sequencing of therapies relative to T-DXd will depend on the magnitude of benefit and the toxicity profile. Meanwhile, several ongoing trials may further shake up current treatment paradigms in the first-line setting (Table 1). Finally, with the increasing use of pertuzumab and T-DM1 in the early-stage settings, in the future, treatment paradigms for patients presenting with relapsed, treatment-refractory disease vs de novo metastatic breast cancer may become more and more distinct.
The Bottom Line
DESTINY-Breast03 is a practice-changing study and firmly establishes T-DXd in the second-line setting in patients with HER2-positive metastatic breast cancer. Exceptions to this generalization are patients with preexisting interstitial lung disease, and those with brain metastases, in whom tucatinib-capecitabine-trastuzumab could be prioritized, particularly for those patients with active/progressive CNS disease. Ongoing clinical trials of T-DXd in patients with brain metastases and in the first-line setting may further redefine the role of T-DXd moving forward.
DISCLOSURE: Dr. Lin has received institutional research support from Genentech, Merck, Pfizer, Seattle Genetics, AstraZeneca, Zion Pharmaceuticals, and Olema Pharmaceuticals; has served as an advisor or consultant to Artera, Pfizer, Puma, Seattle Genetics, Daiichi Sankyo, AstraZeneca, Prelude Therapeutics, Denali Therapeutics, Olema Pharmaceuticals, Aleta BioPharma, Affinia Therapeutics, and Voyager Therapeutics; and has received royalties from UpToDate.
REFERENCES
1. Slamon DJ, Leyland-Jones B, Shak S, et al: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001.
2. Swain SM, Miles D, Kim SB, et al: Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 21:519-530, 2020.
3. Verma S, Miles D, Gianni L, et al: Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783-1791, 2012.
4. Vici P, Pizzuti L, Michelotti A, et al: A retrospective multicentric observational study of trastuzumab emtansine in HER2 positive metastatic breast cancer: A real-world experience. Oncotarget 8:56921-56931, 2017.
5. Baselga J, Cortes J, Kim SB, et al: Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109-119, 2012.
6. Giordano SH, Temin S, Chandarlapaty S, et al: Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 36:2736-2740, 2018.
7. Modi S, Saura C, Yamashita T, et al: Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610-621, 2020.
8. Murthy RK, Loi S, Okines A, et al: Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 382:597-609, 2020.
9. Saura C, Oliveira M, Feng YH, et al: Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Phase III NALA trial. J Clin Oncol 38:3138-3149, 2020.
10. Rugo HS, Im SA, Cardoso F, et al: Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: A phase 3 randomized clinical trial. JAMA Oncol 7:573-584, 2021.
11. Cortes J, Kim SB, Chung WP, et al: Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med 386:1143-1154, 2022.
12. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Antiemesis. Version 2.2022. Available at https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed May 2, 2022. Accessed May 5, 2022.
13. Tarantino P, Modi S, Tolaney SM, et al: Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: A review. JAMA Oncol 7:1873-1881, 2021.
14. Gennari A, Andre F, Barrios CH, et al: ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 32:1475-1495, 2021.
15. Hurvitz S, Kim SB, Chung WP, et al: Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients with HER2+ metastatic breast cancer: Subgroup analyses from the randomized phase 3 study DESTINY-Breast03. 2021 San Antonio Breast Cancer Symposium. Abstract GS3-01. Presented December 9, 2021.
16. Kabraji S, Ni J, Sammons S, et al: Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. 2021 San Antonio Breast Cancer Symposium. Abstract PD4-05. Presented December 8, 2021.
17. Vaz Batista M, Cortez P, Ruiz M, et al: Trastuzumab deruxtecan in patients with HER2[+] or HER2-low-expressing advanced breast cancer and central nervous system involvement: Preliminary results from the DEBBRAH phase 2 study. 2021 San Antonio Breast Cancer Symposium. Abstract PD4-06. Presented December 8, 2021.
18. Bartsch R, Berghoff AS, Furtner J, et al: Intracranial activity of trastuzumab deruxtecan (T-DXd) in HER2-positive breast cancer patients with active brain metastases: Results from the first stage of the phase II TUXEDO-1 trial. ESMO Congress 2021. Abstract 280P. Presented September 16, 2021.
19. Lin NU, Murthy RK, Abramson V, et al: Updated results of tucatinib vs placebo added to trastuzumab and capecitabine for patients with previously treated HER2-positive metastatic breast cancer with brain metastases (HER2CLIMB). 2021 San Antonio Breast Cancer Symposium. Abstrct PD4-04. Presented December 8, 2021.

