Patients with ultra-low–risk breast cancer, as classified by the MammaPrint 70-gene assay, had “excellent” long-term outcomes regardless of clinical risk or receipt of adjuvant therapy, a new analysis of the MINDACT trial has shown.1 In a separate study, a retrospective analysis of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-42 trial used the 70-gene signature to determine which patients with ultra-low–risk disease might benefit from extended endocrine therapy.2

“The 70-gene signature can identify patients with an ultra-low risk of recurrence, and these patients could be candidates for further de-escalation of treatments….”— Josephine Lopes Cardozo, MD
Tweet this quote
From MINDACT, 8-year breast cancer–specific survival was 99.2% among patients classified as clinically high risk but ultra-low risk by the MammaPrint 70-gene assay and 99.7% for the genomically ultra-low–risk and clinically low-risk patients, reported Josephine Lopes Cardozo, MD, of the Netherlands Cancer Institute in Amsterdam, at the 2021 ASCO Annual Meeting.
The MammaPrint 70-gene signature identifies patients at low or high risk of distant recurrence within 5 years of breast cancer diagnosis. The threshold for ultra-low–risk was defined as cancers with 100% breast cancer–specific survival at 15 years in independent validation cohorts for the 70-gene signature. This group was further evaluated in the current study.
“Patients with an ultra-low–risk 70-gene signature had an excellent prognosis, with 8-year breast cancer–specific survival rates above 99%, regardless of clinical risk,” said Dr. Cardozo. “Very few patients developed distant metastases. Furthermore, we observed excellent distant metastasis–free interval rates for the 84% of ultra-low–risk patients who received endocrine therapy alone or no adjuvant systemic treatment.”
She added: “Our results confirm previously published results of excellent survival in ultra-low–risk patients in the largest cohort to date…. The 70-gene signature can identify patients with an ultra-low risk of recurrence, and these patients could be candidates for further de-escalation of treatments, thus further reducing overtreatment and the risk of side effects,” she added.
MINDACT Analysis of Ultra-Low–Risk Subset
The phase III MINDACT trial of 6,693 patients with early breast cancer established the 70-gene signature’s ability to risk-stratify patients by a tumor’s genomic signature.3 The study’s primary finding was that many patients with clinically high-risk/genomically low-risk tumors can safely avoid chemotherapy.
“Long-term follow-up will reveal whether the excellent prognosis of ultra-low–risk patients continues, as was seen in other studies.”— Josephine Lopes Cardozo, MD
Tweet this quote
Within MINDACT, there were 1,000 patients considered ultra-low risk by genomic profiling (15% of the total population). This group had many favorable characteristics: two-thirds were older than age 50, 80% had node-negative disease, 96% had grade 1 or 2 tumors, and 97% were hormone receptor–positive and HER2-negative. Additionally, 16% received no adjuvant systemic therapy, 69% received endocrine therapy, and 14% were treated with chemotherapy. Within the ultra-low–risk group, 741 patients were clinically low risk and 259 were clinically high risk (with mostly larger, higher-grade tumors and positive lymph nodes).
The 8-year results are shown in Table 1, including “excellent” breast cancer–specific survival in all low-risk subsets, Dr. Cardozo reported. In the ultra-low–risk group, the reduction in distant metastasis or breast cancer death at 8 years was 35% lower than for the low-risk group (hazard ratio [HR] = 0.65; 95% confidence interval [CI] = 0.45–0.94; though the confidence intervals are high).
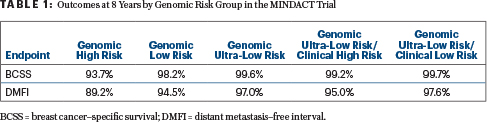
A comparison of treatment for the ultra-low–risk group showed no difference in the risk of breast cancer death or distant metastasis based on chemotherapy (yes vs no, HR = 0.98, 95% CI = 0.37–2.61) or endocrine therapy (yes vs no, HR = 0.59, 95% CI = 0.27–2.13). The distant metastasis–free interval was 97.8% with no adjuvant systemic therapy, 97.4% with adjuvant endocrine therapy alone, and 94.9% in patients who received chemotherapy with or without endocrine therapy.
In closing, Dr. Cardozo noted that low-risk breast cancer can recur very late. “Long-term follow-up will reveal whether the excellent prognosis of ultra-low–risk patients continues, as was seen in other studies,” she said.
70-Gene Signature Applied to NSABP B-42
In a separate study, investigators found the 70-gene signature could suggest which patients might benefit from extended-duration endocrine therapy.2 A substudy of the phase III NSABP B-42 trial, the investigation included patients with early breast cancer who were disease-free after 5 years of adjuvant endocrine therapy and were randomly assigned to 5 more years of letrozole or placebo.
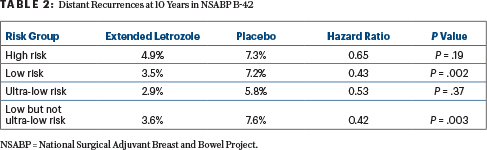
The MammaPrint retrospective analysis included 1,866 of the original 3,903 NSABP B-42 patients, now followed for a median of 10.4 years. The 70-gene signature classified 37.8% of the patients as high risk and 62.2% as low risk and further classified the low-risk patients as ultra-low risk (13.5%) and “low but not ultra-low risk” (48.7%).
Patients classified as high risk by MammaPrint did not benefit significantly from more letrozole, whereas the low-risk group had a 57% reduction in the relative risk of distant relapse at 10 years (Table 2). Low-risk patients but not high-risk patients also benefited from extended letrozole with respect to disease-free survival (HR = 0.67; P < .001) and breast cancer–specific interval (HR = 0.51; P < .001), reported Priya Rastogi, MD, of the University of Pittsburgh.

Priya Rastogi, MD
Further division of the groups showed an effect on distant relapse only in the low- but not ultra-low–risk group and not the ultra-low–risk group (Table 2). Only the low- but not ultra-low–risk group had significant improvements in disease-free survival and breast cancer–free interval (P < .001) as well.
“Genomic classifiers that predict risk of late recurrence and/or benefit from extended endocrine therapy may further assist with the decision to recommend extended aromatase inhibitor therapy,” Dr. Rastogi commented. “Further confirmation in similar data sets of extended endocrine therapy would be important.”
DISCLOSURE: Dr. Cardozo reported no conflicts of interest. Dr. Rastogi has been reimbursed for travel, accommodations, or other expenses by AstraZeneca, Genentech/Roche, and Lilly.
REFERENCES
1. Cardozo JL, et al: Outcome of patients with an ultralow-risk 70-gene signature in the MINDACT trial. 2021 ASCO Annual Meeting. Abstract 500. Presented June 6, 2021.
2. Rastogi P, et al: Utility of the 70-gene MammaPrint assay for prediction of benefit from extended letrozole therapy in the NRG Oncology/NSABP B-42 trial. 2021 ASCO Annual Meeting. Abstract 502. Presented June 6, 2021.
3. Piccart M, et al: 70-gene signature as an aid for treatment decisions in early breast cancer: Updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol 22:476-488, 2021.


