In advanced ovarian cancer, the duration of maintenance bevacizumab should remain 15 months, according to the European multicenter phase III ENGOT/GCIG trial. These results were presented during the 2021 ASCO Annual Meeting by Jacobus Pfisterer, MD, PhD, of the AGO Study Group and Gynecologic Oncology Center in Kiel, Germany.1
“Although median progression-free survival of about 2 years was longer than in the original trials [of maintenance bevacizumab], longer treatment with bevacizumab improved neither progression-free nor overall survival in patents with primary epithelial ovarian, follicular tube, or primary peritoneal cancer. The bevacizumab treatment duration of 15 months as part of the first-line treatment of advanced ovarian cancer remains the standard of care,” Dr. Pfisterer said.

Jacobus Pfisterer, MD, PhD

Rachel Grisham, MD
“This is a really important study that nicely answers the question about the optimal duration of bevacizumab in the upfront setting,” said session co-moderator Rachel Grisham, MD, of Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York.
Background and Study Rationale
The GOG-2182 and ICON7/AGO-OVAR 113 trials revealed that the early and continuous addition of bevacizumab for 15 months and 12 months, respectively, to standard carboplatin/paclitaxel significantly improved progression-free survival. In both studies, the maximal benefit was observed at the time point of the highest cumulative bevacizumab exposure—immediately after the last bevacizumab cycle. However, the optimal bevacizumab duration was never clearly established, so the current randomized phase III ENGOT/GCIG trial examined whether prolonging bevacizumab for up to 30 months would improve its efficacy, explained Dr. Pfisterer.
About the ENGOT/GCIG Trial
The study enrolled 927 women from 161 centers with stage IIb to IV epithelial ovarian (84%), fallopian tube, or peritoneal cancer. Patients underwent primary cytoreductive surgery followed by six cycles of chemotherapy (paclitaxel at 175 mg/m2 plus carboplatin AUC 5) and bevacizumab (15 mg/kg) every 3 weeks. After induction, they were randomly assigned to receive bevacizumab for either the standard 15 months or 30 months, which was the experimental arm.
The primary endpoint was investigator-assessed progression-free survival. The trial was designed with 80.2% power to detect a hazard ratio of 0.66 favoring 30 months of maintenance bevacizumab, after 697 progression-free survival events.
Baseline characteristics were balanced between the arms; median age was 61 years, 96% had an Eastern Cooperative Oncology Group performance status of 0 or 1, 58% had no residual tumor, and 77% had high-grade serous histology.
Key Results and Toxicity
The study found no significant benefit to extending bevacizumab maintenance beyond 15 months to 30 months, as shown in Table 1.
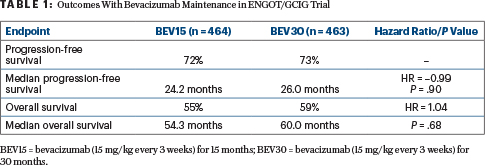
Subgroup analyses examined outcomes in patients with FIGO (International Federation of Gynecology and Obstetrics) stage IIB to IIIC disease and no residual tumor as well as in patients with FIGO stage IIB to IIIC with residual tumor or FIGO stage IV disease. Median progression-free survival was approximately 38 months in the first group and approximately 19 months in the second group, with no significant differences between the treatment arms, Dr. Pfisterer reported.
“Due to evidence of a nonproportional distribution of events,” he said the investigators performed restricted mean analyses of all those endpoints. They were all consistent with the initial results.
Grade 3 to 5 adverse events were observed in 63% of patients treated for 15 months and in 68% receiving 30 months of bevacizumab. Grade 5 events occurred in eight patients and two patients, respectively. Serious adverse events of special interest were seen in 32% and 38%, respectively, including grade ≥ 3 hypertension in 20% of the 15-month arm and 25% of the 30-month arm; other serious adverse events were rare, occurring in less than 5% per arm; they included thromboembolism, fistula, gastrointestinal perforation, proteinuria, hemorrhage, and myocardial infarction.
DISCLOSURE: The study was conducted by F. Hoffmann–La Roche Ltd. Dr. Pfisterer has received honoraria from Amgen, AstraZeneca, Axion Healthcare Strategies, Bionest Partners, Chugai Pharma, Clovis Oncology, Decision Resources, GSK, iMed Institut, Juniper, Medupdate, MSD Oncology, ProSapient, Roche Pharma AG, SAI MedPartners, Simon-Kucher & Partners, Tesaro, Teva, and Vox Bio; has served as a consultant or advisor to AstraZeneca, Clovis Oncology, MSD Oncology, Roche Pharma AG, and Tesaro; has received institutional research funding from Roche Pharma AG and Tesaro; and has been reimbursed for travel, accommodations, or other expenses by Roche Pharma AG. Dr. Grisham has received personal fees from Clovis, Mateon, Regeneron, Verastem, Amgen, Medscape, Aptitude Health, PER, Signatera, and GSK, outside the submitted work.
REFERENCES
1. Pfisterer J, Joly F, Kristensen G, et al: Optimal treatment duration of bevacizumab combined with carboplatin and paclitaxel in patients with primary epithelial ovarian, fallopian tube, or peritoneal cancer. 2021 ASCO Annual Meeting. Abstract 5501. Presented June 7, 2021.
2. Burger RA, Brady MF, Bookman MA, et al: Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011.
3. Perren TJ, Swart AM, Pfisterer J, et al: A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484-2496, 2011. Erratum: N Engl J Med 366:284, 2012.

