The much-anticipated SWOG S1505 trial has failed to show that one preoperative regimen is better than another in resectable pancreatic cancer.1 “Perioperative modified FOLFIRINOX and gemcitabine/nab-paclitaxel appear to have similar efficacy, with acceptable safety and resectability rates,” announced Davendra Sohal, MD, MPH, of the University of Cincinnati, Ohio, during the ASCO20 Virtual Scientific Program. Perhaps more surprising, neither regimen led to an improvement in 2-year survival over historical rates, he added.

“Rapid accrual in this trial demonstrates the growing interest in preoperative chemotherapy in pancreatic cancer.”— Davendra Sohal, MD, MPH
Tweet this quote
Resection followed by adjuvant chemotherapy remains the standard of care for resectable pancreatic cancer, but there has been increasing interest in neoadjuvant treatment. “Outcomes remain suboptimal, perhaps due to the inability of many patients to receive adjuvant chemotherapy…. We now know clearly from preclinical and clinical work that pancreatic cancer is a systemic disease from the outset,” Dr. Sohal said.
SWOG S1505 Details
To assess the potential of early control of systemic disease with multiagent perioperative chemotherapy, the randomized phase II SWOG S1505 trial compared modified FOLFIRINOX (fluorouracil [5-FU] at 2,400 mg/m2 over 46 hours, irinotecan at 180 mg/m2, and oxaliplatin at 86 mg/m2) to gemcitabine (1,000 mg/m2) plus nab-paclitaxel at 125 mg/m2 in patients with resectable pancreatic ductal carcinoma (modification included the elimination of 5-FU bolus and leucovorin). Resectability was strictly defined by established criteria seen on enhanced imaging.
Patients received 12 weeks of preoperative chemotherapy and then were restaged to establish the absence of progressive disease; eligible patients underwent resection and then 12 weeks of adjuvant treatment. Growth factors were not given unless neutropenia developed.
The primary outcome was 2-year overall survival, using a “pick-the-winner” design. Investigators first compared 2-year overall survival for each arm with the historical threshold of 40%. If 2-year survival reached 58% or more, they then planned to compare the two arms to determine the better treatment, explained Dr. Sohal.
Of 147 patients who enrolled, 44 proved to be ineligible (ie, unresectable) by radiology review and 1 other was unevaluable, leaving 102 eligible patients for the final efficacy analysis: 55 treated with modified FOLFIRINOX and 47 treated with gemcitabine/nab-paclitaxel.
Preoperative chemotherapy was completed by 85% of patients in each arm. Resection was performed on 73% of the modified-FOLFIRINOX arm and 70% of the gemcitabine/nab-paclitaxel arm. A total of 55% of patients in each arm started adjuvant therapy, with 49% and 40%, respectively, completing all treatment.
Similar Efficacy Between Arms
The 2-year overall survival was 41.6% with modified FOLFIRINOX (P = .42) and 48.8% with gemcitabine/nab-paclitaxel (P = .12)—a finding that missed the target rate of 58% at 2 years. Median overall survival was 22.4 months and 23.6 months, respectively (Table 1), Dr. Sohal reported. Median disease-free survival after resection was 10.9 months with modified FOLFIRINOX and 14.2 months with gemcitabine/nab-paclitaxel (P = .87).
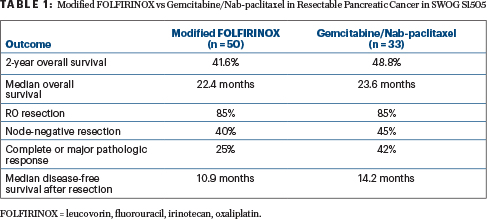
“Neither arm’s 2-year overall survival estimate was statistically significantly higher than the a priori [historical] threshold of 40%,” he noted.
Surgical Results
For patients who underwent resection, negative surgical margins were achieved by 85% in each arm; 40% of those given modified FOLFIRINOX and 45% of those given gemcitabine/nab-paclitaxel had a node-negative resection; and 25% and 42%, respectively, obtained a complete or major pathologic response.
Disease-free survival was somewhat higher in the gemcitabine/nab-paclitaxel arm (14.2 months vs 10.9 months), and patients in this arm were more likely to have node-negative resections. “This may have compensated for the somewhat lower complete rate for all treatment in this arm, ultimately leading to equivalent overall survival in the two arms,” Dr. Sohal suggested. However, overall “there is little evidence that either regimen improves overall survival compared with the historical standard,” he concluded.
Adverse Events
Grade 3 or 4 adverse events were as expected, with the most common being hematologic toxicity, fatigue, diarrhea, nausea, and neuropathy. In the preoperative setting, a slightly higher proportion of the gemcitabine/nab-paclitaxel arm experienced neutropenia (38% vs 19%), whereas a somewhat higher proportion in the modified-FOLFIRINOX arm had diarrhea (17% vs 9%) and nausea (8% vs 2%).
In the postoperative setting, the rates of grade 3 or 4 adverse events were lower overall, likely due to fewer patients receiving chemotherapy and to reduced doses carried over from the preoperative period, explained Dr. Sohal. A higher proportion of patients in the gemcitabine/nab-paclitaxel arm experienced neutropenia (27% vs 0%) and a higher proportion on modified FOLFIRINOX developed neuropathy (16% vs 4%).
“Rapid accrual in this trial demonstrates the growing interest in preoperative chemotherapy in pancreatic cancer,” shared Dr. Sohal. “This study also showed the importance of central radiology review in future studies of potentially resectable cancer, to ensure eligibility according to the protocol.”
DISCLOSURE: Dr. Sohal has received honoraria from Foundation Medicine; has served as a consultant or advisor to Ability Pharma, Perthera, and PierianDx; and has received institutional research funding from numerous companies.
REFERENCE
1. Sohal D, Duong MT, Ahmad SA, et al: SWOG S1505: Results of perioperative chemotherapy with mFOLFIRINOX versus gemcitabine/nab-paclitaxel for resectable pancreatic ductal adenocarcinoma. ASCO20 Virtual Scientific Program. Abstract 4504.



