The addition of the CD38 antibody isatuximab-irfc to carfilzomib and dexamethasone nearly halved the risk of disease progression or death in patients with relapsed or refractory multiple myeloma, the interim analysis of the phase III IKEMA trial has shown.1
“Isatuximab plus carfilzomib and dexamethasone [Kd] showed a significant improvement in progression-free survival, with a 47% reduction in risk vs Kd alone and a clinically meaningful improvement in depth of response,” said Philippe Moreau, MD, of the University Hospital Hôtel-Dieu, Nantes, France. “Isatuximab plus Kd was well tolerated, with manageable safety and a favorable benefit-risk profile. The triplet regimen represents a possible new standard-of-care treatment in patients with relapsed myeloma.”

“The triplet regimen [isatuximab, carfilzomib, dexamethasone] represents a possible new standard-of-care treatment in patients with relapsed myeloma.”— Philippe Moreau, MD
Tweet this quote
The benefit of the triplet combination was observed across subgroups, including patients with high-risk cytogenetics or the elderly,” he added in his late-breaking presentation during EHA25 Virtual Congress, the virtual edition of the 25th European Hematology Association Annual Congress. After a median follow-up of 20.7 months, median progression-free survival, the primary endpoint, had not been reached for patients treated with isatuximab plus Kd, but it was 19.5 months for patients treated with Kd alone (hazard ratio [HR] = 0.531; P = .0007), Dr. Moreau reported.
Isatuximab is an immunoglobulin G1 monoclonal antibody targeting a CD38 transmembrane glycoprotein on multiple myeloma cells, with a mechanism of action similar to that of the anti-CD38 antibody daratumumab. Isatuximab is approved in the United States and Europe in combination with pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma after at least two prior lines of therapy.
IKEMA Details
In the phase III IKEMA trial, 302 patients with relapsed or refractory myeloma previously treated with one to three prior lines of therapy were randomly assigned 3:2 to receive isatuximab plus Kd or Kd alone. Isatuximab was given at 10 mg/kg weekly for 4 weeks and then every 2 weeks. Carfilzomib was given at 20 mg/m2 on days 1 and 2 and then at 56 mg/m2 thereafter twice weekly for 3 of 4 weeks; dexamethasone was given at 20 mg twice weekly. Treatment was continued until disease progression or unacceptable toxicity. The primary endpoint was progression-free survival by independent review.
At a prespecified interim analysis, the trial met its primary endpoint, showing a 47% reduction in the risk of disease progression or death. A subgroup analysis showed “a consistent treatment effect for isatuximab plus Kd across subgroups,” said Dr. Moreau. These groups included the following patients: aged 65 and older, those with baseline estimated glomerular filtration rates up to 60 mL/min/1.73 m2, those with more than one prior line of therapy, those who had not previously received a proteasome inhibitor or immunomodulatory agent, those with high-risk cytogenetic status, and those with revised International Scoring System stage II at study entry.
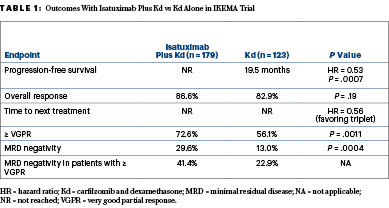
Overall response rates were similar between the arms; however, isatuximab plus Kd resulted in “deeper responses, consistent with a striking progression-free survival improvement,” Dr. Moreau said (Table 1). “Minimal residual disease negativity with isatuximab plus Kd was approximately 30% in the intent-to-treat population.” Overall survival data were not mature at the time of data cutoff.
Similar Tolerability
Grade ≥ 3 treatment-emergent adverse events occurred in 76.8% of patients treated with the triplet and 67.2% of those treated with Kd. Grade ≥ 3 cardiac failure occurred in seven patients treated with the triplet and five treated with Kd. Grade ≥ 3 hematologic toxicities included, respectively, anemia (22.0% vs 17.7%), neutropenia (19.2% vs 7.4%), and thrombocytopenia (29.9% vs 23.8%). Infusion reactions occurred mainly during the first infusion and were mostly grade 1 or 2.
KEY POINTS
- In the phase III IKEMA trial, the triplet of isatuximab, carfilzomib, and dexamethasone reduced the risk of disease progression or death by 47% in patients with relapsed or refractory multiple myeloma.
- The triplet also deepened responses significantly and doubled the rate of minimal disease negativity.
“Despite more grade 3 or higher treatment-emergent adverse events, the addition of isatuximab to Kd did not increase mortality, serious treatment-related adverse events, or events leading to treatment discontinuations,” Dr. Moreau indicated.
DISCLOSURE: The study was sponsored by Sanofi. Dr. Moreau has received honoraria from Amgen, Celgene, GSK, Janssen-Cilag, Novartis, and Takeda and has served as a consultant or advisor to Amgen, Celgene, GSK, Janssen, and Takeda.
REFERENCE
1. Moreau P, Dimopoulos MA, Mikhael J, et al: Isatuximab plus carfilzomib and dexamethasone vs carfilzomib and dexamethasone in relapsed/refractory multiple myeloma (IKEMA): Interim analysis of a phase III, randomized, open-label study. EHA25 Virtual Congress. Abstract LB2603.

