As part of The ASCO Post’s coverage of the 2019 ASCO Annual Meeting, featured here are summaries of five abstracts of different clinical trials evaluating newer treatments for follicular and marginal zone lymphomas as well as diffuse large B-cell lymphoma.

Syed Ali Abutalib, MD

Sonali M. Smith, MD, FASCO
Follicular and Marginal Zone Lymphomas
ABSTRACT 7513: Multicenter nonregistration phase IIIb study of induction therapy with lenalidomide and rituximab (R2) followed by maintenance in relapsed or refractory follicular and marginal zone lymphomas (MAGNIFY)1
Background: There is no single standard of care for patients with relapsed or refractory follicular or marginal zone lymphomas. Lenalidomide has activity as monotherapy and in combination with anti-CD20 monoclonal antibodies. MAGNIFY has induction and maintenance components, with the primary endpoint of the induction component reported here. Induction consisted of 12 months of lenalidomide at 20 mg/d on days 1 to 21 every 28 days with rituximab at 375 mg/m2/week in cycle 1 followed by every 8 weeks from cycle 3 onward. After at least 12 cycles, patients with stable disease or better were randomly assigned evenly to continue R2 vs single-agent rituximab maintenance therapy.
Primary Endpoint: Overall response rate by 1999 International Working Group (IWG) response criteria for induction phase
Results: At a median of 1.3 years of follow-up, 370 patients (80% with grade 1–3a follicular lymphoma; 20% with marginal zone lymphoma) were enrolled; 83% had stage III and IV disease and had received a median of two prior therapies (95% with rituximab). Of note, both patients with rituximab-sensitive and rituximab-refractory disease were included.
The results of efficacy-evaluable patients are shown in Table 1.
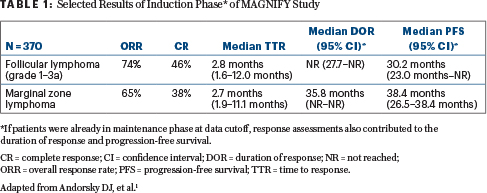
Although patients with rituximab-sensitive and rituximab-refractory disease had similar response rates, the duration of response and progression-free survival were not reached in the rituximab-sensitive group. The most common all-grade adverse events were fatigue, neutropenia, diarrhea, nausea, and constipation. The incidence of grade 3 and 4 neutropenia was 34%; the incidence of all other grade 3 and 4 adverse events was less than 6%.
Clinical Implications: The MAGNIFY study shows R2 as induction therapy is active, with a tolerable safety profile, in patients with relapsed or refractory follicular and marginal zone lymphomas and in those with disease refractory to rituximab. On May 28, 2019, the U.S. Food and Drug Administration approved R2 in these patient populations for up to 12 cycles.2 About 142 of 370 patients have entered the ongoing maintenance phase of the trial.
ABSTRACT 7514: Efficacy and time to next treatment following R2 (n = 178) or placebo plus rituximab (n = 180) in patients with relapsed or refractory follicular and marginal zone lymphomas (AUGMENT)3
Methods: The AUGMENT phase III study evaluated patients with relapsed or refractory follicular (grade 1–3a; 82%) and marginal zone lymphomas (18%) after at least one prior systemic therapy. In contrast to the MAGNIFY trial, here all patients had rituximab-sensitive disease. Patients were randomly assigned 1:1 to R2 (lenalidomide at 20 mg/d, days 1–21 every 28 days for 12 cycles and intravenous rituximab at 375 mg/m2/week x 4 infusions in cycle 1 followed by day 1 of cycles 2–5) vs placebo plus rituximab (same dosing schedule). Note the difference in schedule from the MAGNIFY study.
Endpoints: The primary endpoint is progression-free survival using 2007 IWG criteria (which includes positron-emission tomography, immunohistochemistry, and flow cytometry for definitions of response), and secondary/exploratory analyses were time to next antilymphoma/chemotherapy treatment and response to next treatment.
Results: The median progression-free survival was significantly superior for R2 over rituximab/placebo (39.4 vs 14.1 months; hazard ratio [HR] = 0.46; P < .0001). As of June 22, 2018, the median time to next antilymphoma/chemotherapy treatment and second progression-free survival were not reached for R2 and were significantly longer than with placebo plus rituximab (HR = 0.54, 0.50, and 0.52, respectively). For 49 of 178 patients treated with R2 and 80 of 180 patients treated with placebo plus rituximab receiving next antilymphoma therapy, the response was generally better with R2 (57% objective response rate and 31% complete remission rate vs 36% and 16% with rituximab/placebo, respectively).
Clinical Implications: The AUGMENT study suggests that R2 (vs rituximab/placebo) prolonged the time to subsequent treatment and is associated with a significantly longer second progression-free survival. This is one of the largest trials to show the relatively modest activity of rituximab monotherapy in the relapsed setting. Although there are small differences in the schedule and number of rituximab doses for the R2 regimen, both the AUGMENT and MAGNIFY trials show significant activity of R2 in this setting. Both trials have led to the approval of R2 in patients with these types of lymphoma. Presently, we prefer the R2 regimen, as developed in the AUGMENT trial.
Diffuse Large B-Cell Lymphoma
ABSTRACT 7521: Update of the single-arm phase II study of MOR208 [an Fc-enhanced, humanized, anti-CD19 monoclonal antibody] and lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (DLBCL): Response rates in subgroups of patients with an extremely poor prognosis (L-MIND study)4
Primary Endpoint: Independent review committee–assessed objective response rate
Methods: Key inclusion criteria were adequate organ function, up to three prior lines of therapy (including at least one anti-CD20 therapy), and ineligibility for hematopoietic cell transplantation.
Results: Investigator-assessed complete and partial response rates were 33% and 25%, respectively, with an objective response rate of 58%, comparable to the independent review committee assessment (objective response rate, 54%; complete response rate, 32%). The objective response rate was 46% in patients with at least two prior therapies, 59% in rituximab-refractory patients, 56% in those with last treatment–refractory disease, 58% in patients who had an early relapse, 57% in patients with a baseline International Prognostic Index (IPI) of 3 to 5, 71% in patients with non–germinal center B-cell–like (non-GCB), and 53% in those with GCB disease. Investigator-assessed median progression-free survival and overall survival (intention-to-treat analysis) were 16.2 months (95% confidence interval [CI] = 6.3 to not reached) and not reached (95% CI = 18.6 to not reached), respectively.
Clinical Implications: Overall, the therapy was well tolerated: 72% of patients stayed on lenalidomide at a dose of at least 20 mg/d. Treatment-related serious adverse events, mainly infections or neutropenic fever, occurred in 17% of patients. In the L-MIND study, MOR208 plus lenalidomide showed encouraging activity in this patient population. The preferential activity of the combination in non-GCB disease is consistent with lenalidomide monotherapy data.
One of the challenges for this study and for other trials in this space is the approval and availability of chimeric antigen receptor (CAR) T-cell therapy. Patient selection for CAR T-cell therapy vs other options (including this regimen, polatuzumab vedotin plus bendamustine/rituximab, or others) is in evolution, with no clear guidelines to date.
ABSTRACT 7507: Rituximab maintenance (vs observation) for patients with DLBCL in first complete remission: Results of second randomization of HOVON-Nordic Lymphoma Group phase III study5
Methods: Patients in first complete remission after rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) were randomly assigned to 24 months of rituximab maintenance (intravenous at 375 mg/m2 every 8 weeks; n = 199) or observation (n = 199). A total of 48% were 66 years or older, and 49% were male.
Endpoints: The primary endpoint was disease-free survival from maintenance randomization. Secondary endpoints were overall survival and adverse events.
Results: The majority of patients (54%) had a high-intermediate- or high–age-adjusted International Prognostic Index (IPI) score. After a median follow-up of 79.9 months (maximum, 125.7 months), the 5-year disease-free survival rate was 79% for rituximab maintenance vs 74% for observation. This difference was not statistically significant, with a hazard ratio of 0.83 (95% Cl = 0.57–1.19, P = .31, adjusted for age and age-adjusted IPI). Overall survival also did not differ significantly (85% vs 83% at 5 years).
“Rituximab maintenance therapy provides no additional benefit for patients with DLBCL in first complete remission after standard R-CHOP therapy.”— Syed Ali Abutalib, MD, and Sonali M. Smith, MD, FASCO
Tweet this quote
Clinical Implications: Once again, the ability to improve upon standard R-CHOP has been unsuccessful. Several groups have evaluated strategies after R-CHOP, including consolidative autologous stem cell transplant, maintenance with oral agents, or maintenance rituximab. This study definitively shows that rituximab maintenance therapy provides no additional benefit for patients with DLBCL in first complete remission after standard R-CHOP therapy. No clinical subgroup benefited from rituximab maintenance.
ABSTRACT 7508: Smart Start: Final results of rituximab, lenalidomide, and ibrutinib lead-in prior to combination with chemotherapy for patients with newly diagnosed non-GCB (as defined by Hans criteria) DLBCL: A single-arm phase II study6
Background and Methods: Anthracycline-based chemotherapy has been the standard of care for front-line DLBCL for over 4 decades. Although many trials augment R-CHOP, this trial tested the efficacy and safety of a window “chemotherapy-free” approach using lenalidomide, rituximab, and ibrutinib followed by six cycles of either R-CHOP or rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin.
The primary objectives were to determine the objective response rate with two cycles of rituximab (intravenous at 375 mg/m2 on day 1), lenalidomide (25 mg on days 1–10 of 21 days), and ibrutinib (560 mg daily) as initial therapy and the complete response rate after 6 cycles of chemotherapy combined with rituximab, lenalidomide, and ibrutinib. All patients had non-GCB disease as part of eligibility.
Results: Of 60 patients, 58 were evaluable for disease response to the induction/window component. The median patient age was 64 years (28% were at least age 70), and 50% were female. Half the patients had a poor-risk IPI, 61% had advanced-stage disease, and 71% had a Ki67 index of at least 80%. The objective response rate with two cycles of rituximab, lenalidomide, and ibrutinib was 84.6%, and the complete response rate was 38.5%. One patient deferred the preplanned chemotherapy after achieving a complete response to two cycles of rituximab, lenalidomide, and ibrutinib and remains relapse-free after 18 months. The objective response rate after the chemotherapy component exceeded 95%.
Clinical Implications: The Smart Start trial is one of the first window trials to show feasibility and activity of a nonchemotherapy approach using rituximab, lenalidomide, and ibrutinib in treatment-naive DLBCL. Both lenalidomide and ibrutinib have preferential single-agent activity in relapsed or refractory non-GCB disease, and limiting treatment to this subset likely enhanced the observed response rate. It is notable that significant toxicities seen in other trials testing ibrutinib plus R2, such as severe rash, were not observed in this trial. Additional studies evaluating more cycles of rituximab, lenalidomide, and ibrutinib with less chemotherapy consolidation are planned. ■
Dr. Abutalib is Associate Director, Hematology and Cellular Therapy Program; Director, Clinical Apheresis Program, Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Roseland Franklin University of Medicine and Science; Founder and Co-Editor, Advances in Cell and Gene Therapy. Dr. Smith is Elwood V. Jensen Professor of Medicine; Interim Chief, Section of Hematology/Oncology; Director, Lymphoma Program, The University of Chicago.
DISCLOSURE: Dr. Abutalib is an advisor for AstraZeneca. Dr. Smith has served as a consultant or advisor for AbbVie/Genentech, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Gilead Sciences, Kite Pharma, Pharmacyclics, Portola Pharmaceuticals, Seattle Genetics, and TG Therapeutics; and has received research funding from Acerta Pharma/AstraZeneca, Celgene, and Pharmacyclics/Janssen.
REFERENCES
1. Andorsky DJ, Coleman M, Yacoub A, et al: MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. 2019 ASCO Annual Meeting. Abstract 7513. Presented June 3, 2019.
2. U.S. Food and Drug Administration: FDA approves lenalidomide for follicular and marginal zone lymphoma. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenalidomide-follicular-and-marginal-zone-lymphoma. Accessed June 28, 2019.
3. Gribben JG, Izutsu K, Fowler NH, et al: Efficacy and time to next treatment following lenalidomide/rituximab (R2) or rituximab/placebo in patients with R/R indolent NHL (AUGMENT). 2019 ASCO Annual Meeting. Abstract 7514. Presented June 3, 2019.
4. Maddocks KJ, Duell J, Barca EG, et al: Update of the single-arm phase II L-MIND study of MOR208 + lenalidomide in relapsed/refractory diffuse large B-cell lymphoma: Response rates in patient subgroups with poor prognosis. 2019 ASCO Annual Meeting. Abstract 7521. Presented June 3, 2019.
5. Lugtenburg EJ, Brown P, van der Holt B, et al: Rituximab maintenance for patients with diffuse large B-cell lymphoma in first complete remission: Results from a randomized HOVON-Nordic Lymphoma Group phase III study. 2019 ASCO Annual Meeting. Abstract 7507. Presented June 4, 2019.
6. Westin J, Nastoupil LJ, Fayad L, et al: Smart start: Final results of rituximab, lenalidomide, and ibrutinib lead in prior to combination with chemotherapy for patients with newly diagnosed diffuse large B-cell lymphoma. 2019 ASCO Annual Meeting. Abstract 7508. Presented June 4, 2019.

