A handful of investigational drugs in early-phase trials always create a buzz at ASCO Annual Meetings. Two that garnered attention this year, and could eventually change outcomes in the clinic, are the first-in-class KRAS inhibitor AMG 510 and the ROS1 inhibitor repotrectinib. Should late-phase clinical trials confirm early findings, clinicians will certainly welcome these agents in the treatment of some of their patients with non–small cell lung cancers (NSCLCs).
First KRAS Inhibitor Yields Strong Data
Half of heavily pretreated patients with KRAS G12C–positive advanced NSCLC responded to treatment with the KRAS G12C inhibitor AMG 510, in a phase I study.1 “AMG 510 has been safe and well tolerated at dose levels tested in 35 patients in dose exploration. Preliminary monotherapy antitumor activity in KRAS G12C–positive NSCLC was observed,” said Marwan Fakih, MD, of City of Hope, Duarte, California.
The ongoing first-in-human, multicenter, open-label phase I study includes, to date, 35 patients with locally advanced or metastatic KRAS G12C–positive solid tumors previously treated with at least two lines of standard therapy. This study population includes 14 patients with NSCLC, 19 with colorectal cancer, and 2 with appendiceal cancer.

“Preliminary monotherapy antitumor activity [of AMG 510] in KRAS G12C–positive NSCLC was observed.”— Marwan Fakih, MD
Tweet this quote
Among 10 evaluable patients with NSCLC, 5 patients responded. In the other tumor types, the best response was stable disease, Dr. Fakih reported at the ASCO meeting.
As a potential therapeutic target, KRAS has been actively explored for decades but has remained “undruggable” due to a lack of traditional small-molecule–binding pockets on the protein. Consequently, there are no approved targeted agents for KRAS G12C, which is found in approximately 13% of NSCLCs, 3% of colorectal and appendiceal cancers, and 1% to 3% of other solid tumors.
AMG 510 is exploiting a previously hidden groove on the protein surface. “AMG 510 is a novel, first-in-class, small molecule that specifically and irreversibly inhibits KRAS G12C by locking it in an inactive GDP-bound state,” Dr. Fakih explained.
Study Details on AMG 510
The study initially enrolled two to four patients into four dose cohorts, then additional patients were added once the dose proved to be safe. AMG 510 was administered orally once daily, with dose escalation allowed, until disease progression.
At data cutoff, 5 of 10 evaluable patients with NSCLC achieved a partial response and are still on treatment, and 5 others achieved stable disease. In the other tumor types, 14 of 18 evaluable patients with colorectal or appendiceal cancer achieved stable disease. The duration of treatment among responders and patients with stable disease ranged from approximately 7 weeks to about 25 weeks, Dr. Fakih reported.
NOVEL KRAS AND ROS1 INHIBITORS
- In a phase I study, a first-in-class investigational KRAS inhibitor, AMG 510, showed activity in heavily pretreated patients with NSCLC who had KRAS G12C mutations.
- The response rate was 50%, and the clinical benefit rate was 100%, among 10 evaluable patients with the KRAS G12C mutation.
- In the phase I/II TRIDENT-1 trial, the ROS1 inhibitor repotrectinib produced responses in 82% of ROS1-mutated patients who were naive to tyrosine kinase inhibitors and in 39% who received previous treatment.
Dr. Fakih described two of the responders with NSCLC. One, diagnosed in 2010, experienced disease progression after treatment with chemotherapy, radiation, and nivolumab. In 2018, she started on AMG 510 (180 mg) and experienced a 34% reduction in tumor size. As of the data cutoff, she was continuing on treatment beyond 27 weeks. The second patient was diagnosed in 2013 and had received chemotherapy, erlotinib, nivolumab, dasatinib, and the targeted biologic M3541 before starting AMG 510 (360 mg) in 2018. He remains on treatment past 14 weeks and has seen a 67% reduction in tumor size.
AMG510 administration was well tolerated, with most side effects being grade 1 and 2 in nature. Only two possible treatment-related grade 3 events were recorded; they consisted of grade 3 anemia and grade 3 diarrhea. No treatment-related dose-limiting toxicities were recorded.
TRIDENT-1: Repotrectinib Active in Refractory ROS1-Positive Disease

Byoung Chul Cho, MD, PhD
The oral tyrosine kinase inhibitor repotrectinib demonstrated activity and safety in patients with advanced ROS1 fusion–positive NSCLC, in the phase I/II TRIDENT-1 trial, reported by Byoung Chul Cho, MD, PhD, of Yonsei Cancer Center in Seoul, South Korea.2
Repotrectinib is a next-generation inhibitor of ROS1/TRK/ALK, with a 90-fold greater potency for ROS1 than crizotinib. In the study, treatment with repotrectinib led to responses in 82% of patients who were naive to tyrosine kinase inhibitors and 39% of patients who were pretreated with tyrosine kinase inhibitors. It also showed activity in patients with the tyrosine kinase inhibitor–resistant mutation G2032R (Table 1) and in those with central nervous system involvement.
“The TRIDENT-1 study supports repotrectinib as a potential best-in-class ROS1 agent in advanced NSCLC,” Dr. Cho said.
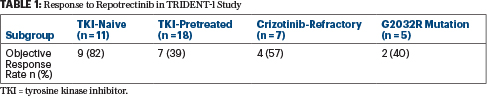
Activity in the 11 patients who were naive to tyrosine kinase inhibitors was especially strong, with responders not yet achieving a median response duration after almost 17 months of follow-up. Individual response durations ranged from about 11 to 18 months in the five patients remaining in response, Dr. Cho reported. “This is exciting, because these are the most promising data presented so far with an ROS1 tyrosine kinase inhibitor in a tyrosine kinase inhibitor–naive patient population,” he noted.
The tyrosine kinase inhibitor was relatively well tolerated, with four dose-limiting toxicity events, which included grade 2 or 3 dizziness in three patients and grade 3 dyspnea and hypoxia in one patient. One grade 5 adverse event was deemed possibly related to the drug.
The phase II portion of TRIDENT-1 is set to begin in 190 patients, including patients both pretreated with and naive to ROS1 inhibitors and patients with NTRK-positive advanced solid tumors. ■
DISCLOSURE: Dr. Fakih has served as a consult/advisor to Amgen, Array BioPharma, and Genentech/Roche; has served on the speakers bureau for Amgen and Taiho Pharmaceutical; and has received research funding from AstraZeneca, Novartis, and Amgen. Dr. Cho reported relationships with TheraCanVac, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Champions Oncology, Dizal Pharma, Dong-A ST, Eli Lilly, Janssen, Mogam Biotechnology Research Institute, MSD, Novartis, Ono, Pfizer, Roche, Takeda, and Yuhan.
REFERENCES
1. Fakih M, O’Neil B, Price TJ, et al: Phase 1 study evaluating the safety, tolerability, pharmacokinetics, and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. 2019 ASCO Annual Meeting. Abstract 3003. Presented June 3, 2019.
2. Cho BC, Drilon AE, Doebele RC, et al: Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). 2019 ASCO Annual Meeting. Abstract 9011. Presented May 31, 2019.

