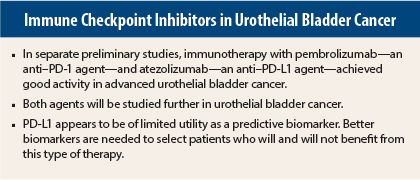
Two immunotherapy agents show promise in preliminary studies of advanced urothelial bladder cancer: the anti–PD-1 (programmed cell death protein 1) antibody pembrolizumab (Keytruda) and the anti–PD-L1 (programmed cell death ligand 1) antibody atezolizumab. Separate phase I studies of each drug showed excellent activity and durable responses in poor-prognosis patients with urothelial bladder cancer and were reported at the 2015 ASCO Annual Meeting.1,2
Metastatic urothelial bladder cancer is associated with poor outcomes. There are no U.S. Food and Drug Administration–approved therapies for relapse after platinum-based therapy, and overall survival is 5 to 7 months in the second-line setting.
Pembrolizumab
Updated safety and efficacy data from the phase Ib KEYNOTE-012 study showed an overall response rate of 27.6%, a disease control rate of 38%, and median overall survival of 12.7 months in a cohort of patients with advanced urothelial bladder cancer treated with pembrolizumab. These findings compare favorably with results of historical trials in this population, noted lead author Elizabeth Plimack, MD, of Fox Chase Cancer Center, Philadelphia.
About 50% of patients in the urothelial bladder cancer cohort were alive at 12 months, and about 85% experienced only mild or no adverse events.
“These results support the further development of pembrolizumab in urothelial bladder cancer and continuing investigation of novel biomarkers,” Dr. Plimack said.
Of 95 patients screened, 62.4% were PD-L1–positive at baseline, according to expression of at least 1% PD-L1–positive tumor cells in tumor nests or a PD-L1–positive band in stroma by a prototype immunohistochemistry assay. A total of 33 patients with PD-L1–positive recurrent or metastatic urothelial bladder cancer were enrolled in the phase Ib trial and treated with pembrolizumab (10 mg/kg every 2 weeks) until complete remission, disease progression, or unacceptable toxicity.
The median age of study patients was 70 years; patients were predominantly male; the majority had Eastern Cooperative Oncology Group (ECOG) 1 performance status; 24% had liver metastasis; and the only metastatic site in 9% was the lymph node. No brain metastasis was allowed. About one-quarter of patients had no prior therapy, and 33% had at least three prior therapies.
Immune-related events occurred in only a few patients; they included colitis, myositis, rhabdomyolysis, rash, and uveitis. Five patients experienced grade 3 or 4 treatment-related adverse events. There was one treatment-related discontinuation.
Radiographic responses were observed in 8 patients (27.6%); 3 (10%) had complete remission; 5 (17.2%) had a partial response; 3 patients (10%) had stable disease; 14 patients (48.3%) had progressive disease; and 4 patients could not be assessed. The disease control rate was 38% (11 patients). Sixty-four percent of patients treated with pembrolizumab had a decrease in target lesions.
The median duration of follow-up was 15 months. The median time to response was 9 weeks; response duration ranged from 8.1 weeks to 64.1 weeks, and some patients are still in response. Three patients remain on therapy.
Median progression-free survival is 2 months. Progression-free survival at 12 months is 19.1%. Median overall survival is 13 months, and 1-year survival is 52.9%.
An investigative analysis of PD-L1 revealed that the best way to maximize detection of response and minimize false-positive results is to measure PD-L1 expression in both tumor cells and tumor-associated inflammatory cells, Dr. Plimack explained.
Among 18 patients with PD-L1–positive tumors in tumor cells only, using a different immunohistochemical method than that used for screening, 33% had an objective response according to RECIST (Response Evaluation Criteria in Solid Tumors) criteria; in 11 PD-L1–negative tumors in tumor cells only, 9% had RECIST response.
According to analysis of PD-L1 expression in both tumor cells and tumor-associated inflammatory cells, the response rate was 29% in 24 PD-L1–postive patients and 0% among 4 PD-L1–negative patients.
Analysis of four gene expression signatures derived from prior studies in melanoma found that a signature related to T-cell receptor signaling was associated with improved clinical benefit and prolonged response, whereas no association was found for the other three gene expression signatures analyzed: interferon gamma, expanded immune, and de novo (33 genes) signatures.
Atezolizumab
Atezolizumab (formerly known as MPDL3280A) had encouraging activity in a cohort of heavily pretreated patients with metastatic urothelial bladder cancer. PD-L1 status, as measured by an SP142 assay, appears to be predictive of benefit but not survival of atezolizumab in urothelial bladder cancer.
“Survival is encouraging, and responses are clinically meaningful in this phase Ia study,” said Daniel P. Petrylak, MD, of Yale Cancer Center, New Haven, Connecticut.
“[Urothelial bladder cancer] is a disease of high mutational complexity and immunogenicity,” Dr. Petrylak explained. “Since PD-L1 is expressed in many tumors, we were justified in doing a phase I trial of atezolizumab in many solid tumors, including triple-negative breast cancer, melanoma, non–small cell lung cancer, renal carcinoma, and [urothelial bladder cancer]. At first, the phase I study enrolled only PD-L1 expressors, and then we took all comers,” he said.
He reported the results of an ongoing dose-expansion phase of the study that included 205 patients who were screened using the highly specific SP142 to measure PD-L1 using four immunohistochemistry scoring levels. Twenty-seven percent of patients had immunohistochemistry 2 or 3 expression (high levels). “We see long-term responses in both low and high expressors of PD-L1,” Dr. Petrylak said.
Adverse events were generally well tolerated, with no treatment-related deaths. Five percent of patients had immune-mediated grade 3-4 events. Overall, 40% had a grade 3 or 4 adverse event of any cause.
Median time to response was 62 days. The overall response rate was 50% for immunohistochemistry 2/3 patients. Patients with low levels of PD-L1 had an overall response rate of 17%. Responders included patients with visceral metastases at baseline, with an overall response rate of 32%. A waterfall plot showed that 44 patients (55%) had a reduced tumor burden. Median duration of treatment has not yet been reached in immunohistochemistry 2/3 patients. Median overall survival is between 10 and 14 months, Dr. Petrylak said. ■
Disclosure: Dr. Plimack has participated in scientific advisory boards for Merck and Genentech, and her institution has received funding from Merck related to the conduct of clinical trials, including the trial featured in this article. Dr. Petrylak has received travel expenses and honoraria from Genentech and research funding from Genentech and Merck.
References
1. Plimack ER, Bellmunt J, Gupta S, et al: Pembrolizumab (MK-3475) for advanced urothelial cancer: Updated results and biomarker analysis for KEYNOTE-012. 2015 ASCO Annual Meeting. Abstract 4502.
2. Petrylak DP, Powles T, Bellmunt J, et al: A phase Ia study of MPDL3280A (anti-PDL1): Updated response and survival data in urothelial bladder cancer. 2015 ASCO Annual Meeting. Abstract 4501.