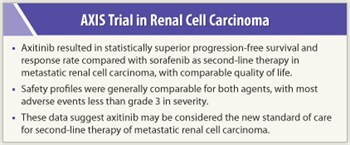
 A randomized comparative effectiveness phase III trial demonstrated significantly superior efficacy for the tyrosine kinase inhibitor axitinib compared to sorafenib (Nexavar) in patients with advanced renal cell carcinoma (RCC). These data suggest that axitinib may become a new standard of care for second-line treatment of metastatic RCC. The findings were presented at the 2011 ASCO Annual Meeting by Brian Rini, MD, of the Cleveland Clinic Taussig Cancer Institute.1
A randomized comparative effectiveness phase III trial demonstrated significantly superior efficacy for the tyrosine kinase inhibitor axitinib compared to sorafenib (Nexavar) in patients with advanced renal cell carcinoma (RCC). These data suggest that axitinib may become a new standard of care for second-line treatment of metastatic RCC. The findings were presented at the 2011 ASCO Annual Meeting by Brian Rini, MD, of the Cleveland Clinic Taussig Cancer Institute.1
Axitinib and AXIS
Axitinib is an oral, selective, next-generation inhibitor of vascular endothelial growth factor receptors (VEGFR)-1, -2, and -3. Compared with first-generation tyrosine kinase inhibitors such as sorafenib, axitinib inhibits VEGFR in vitro at substantially lower concentrations, with little inhibition of other kinases. In phase II trials, axitinib demonstrated activity in patients with cytokine-refractory RCC and sorafenib-refractory RCC, suggesting that it could be an effective therapy for second-line RCC. While sorafenib also had been shown to be effective, no randomized trials had directly compared targeted agents in advanced RCC.
 AXIS was a global randomized phase III trial that enrolled 723 patients who had metastatic RCC with clear cell histology and disease progression following one treatment with a sunitinib (Sutent)-, bevacizumab (Avastin) plus interferon-alfa–, temsirolimus (Torisel)-, or cytokine-based regimen. Key eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, adequate renal, hepatic, and hematologic function, and at least 2 weeks since completion of prior systemic therapy (≥ 4 weeks for bevacizumab/interferon-alfa). Patients were treated with axitinib at 5 mg twice daily, which could be titrated to 7 or 10 mg twice daily; sorafenib was administered at 400 mg twice daily. The primary endpoint was progression-free survival, as assessed by a blinded independent review committee.
AXIS was a global randomized phase III trial that enrolled 723 patients who had metastatic RCC with clear cell histology and disease progression following one treatment with a sunitinib (Sutent)-, bevacizumab (Avastin) plus interferon-alfa–, temsirolimus (Torisel)-, or cytokine-based regimen. Key eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, adequate renal, hepatic, and hematologic function, and at least 2 weeks since completion of prior systemic therapy (≥ 4 weeks for bevacizumab/interferon-alfa). Patients were treated with axitinib at 5 mg twice daily, which could be titrated to 7 or 10 mg twice daily; sorafenib was administered at 400 mg twice daily. The primary endpoint was progression-free survival, as assessed by a blinded independent review committee.
Study Findings
Baseline characteristics were similar among patients on each treatment arm. Based on an intent-to-treat analysis, said Dr. Rini, “axitinib produced a median progression-free survival of 6.7 months, compared to 4.7 months for sorafenib; this corresponded to a stratified hazard ratio of 0.665 with a significant P value of .0001” (Table 1). Significantly greater progression-free survival with axitinib also was seen in patients who received prior cytokines (12.1 vs 6.5 months; P < .0001) or sunitinib therapy (4.8 vs 3.4 months; P = .0107). Forest plot analysis indicated 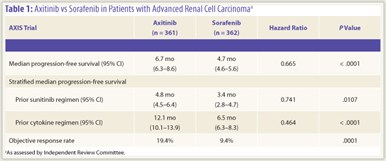 that the survival benefit for axitinib was relatively preserved across patient subgroups when stratified by baseline characteristics and prognostic factors evaluated. The objective response rate was higher with axitinib compared to sorafenib (19.4% vs 9.4%; P = .0001).
that the survival benefit for axitinib was relatively preserved across patient subgroups when stratified by baseline characteristics and prognostic factors evaluated. The objective response rate was higher with axitinib compared to sorafenib (19.4% vs 9.4%; P = .0001).
Patient-reported quality of life was comparable between the two treatment groups. Most adverse events were less than grade 3 in severity.
This head-to-head comparison demonstrates that axitinib provided greater clinical benefit than sorafenib when used as second-line therapy for metastatic RCC, perhaps due to its more potent inhibition of the VEGFR. Results of the AXIS trial, concluded Dr. Rini, suggest that axitinib should be considered the new standard of care for second-line therapy of advanced RCC. ■
Disclosure: Dr. Rini is a consultant for AVEO, GlaxoSmithKline, and Pfizer, and has received research support from GlaxoSmithKline and Pfizer. Dr. Rosenberg is an employee of UBC Scientific Solutions and has previously provided medical writing support, which was funded by Pfizer Inc.
Reference
1. Rini BI, Escudier B, Tomczak P, et al: Axitinib versus sorafenib as second-line therapy for metastatic renal cell carcinoma (mRCC): Results of phase III AXIS trial. 2011 ASCO Annual Meeting. Abstract 4503. Presented June 6, 2011.

 According to Eric Jonasch, MD, of The University of Texas MD Anderson Cancer Center, “These data show that treatment with a potent and specific antiangiogenic agent after immunotherapy does provide significant prolongation of progression-free survival, and that axitinib is clearly superior to...
According to Eric Jonasch, MD, of The University of Texas MD Anderson Cancer Center, “These data show that treatment with a potent and specific antiangiogenic agent after immunotherapy does provide significant prolongation of progression-free survival, and that axitinib is clearly superior to...