For hormone receptor–positive, HER2-negative advanced breast cancer that has progressed on CDK4/6 inhibition plus endocrine therapy, the CDK4/6 inhibitor abemaciclib plus fulvestrant significantly reduced the risk of further disease progression in the phase III postMONARCH study.1
“The postMONARCH trial is the first phase III randomized, placebo-controlled study to show the benefit of continued CDK4/6 inhibition and switching the endocrine therapy beyond [disease] progression on a CDK4/6 inhibitor,” said Kevin Kalinsky, MD, MS, Director of the Glenn Family Breast Center at Winship Cancer Institute of Emory University, Atlanta. “We saw a 27% reduction in the risk of developing a progression-free survival event despite the control arm performing better than expected…. We saw benefit across multiple prespecified and clinically relevant subgroups…. And we saw improvement across key secondary efficacy endpoints, including progression-free survival by blinded independent central review and response.”

Kevin Kalinsky, MD, MS
“Thus, abemaciclib plus fulvestrant offers a targeted therapy option after disease progression on a CDK4/6 inhibitor for patients with hormone receptor–positive, HER2-negative advanced breast cancer not selected for biomarker status,” said Dr. Kalinsky, who presented the findings at the 2024 ASCO Annual Meeting.1
As Dr. Kalinsky explained, CDK4/6 inhibition plus endocrine therapy is the standard first-line treatment of hormone receptor–positive, HER2-negative advanced breast cancer. When these patients experience disease progression, the optimal next treatment remains unclear. Real-world evidence suggests that use of abemaciclib after disease progression on a prior CDK4/6 inhibitor may extend progression-free survival, although phase II trials with CDK4/6 inhibitors aside from abemaciclib have generated mixed results. Targeted therapy options are primarily confined to biomarker-positive patients, ie, those with ESR1 mutations or alterations in the PI3K pathway. The phase III postMONARCH trial aimed to help inform this issue by evaluating the use of abemaciclib plus fulvestrant.
Key Study Results
The postMONARCH trial was a global study that randomly assigned 368 patients to receive abemaciclib or placebo plus fulvestrant. Virtually all patients had experienced disease progression on a CDK4/6 inhibitor plus an aromatase inhibitor as initial therapy for advanced disease; only three participants had relapsed on or after adjuvant therapy with a CDK4/6 inhibitor plus endocrine therapy for early disease. The prior CDK4/6 inhibitor was palbociclib in 59%, ribociclib in 33%, and abemaciclib in 8%. The primary endpoint was investigator-assessed progression-free survival.
With 70% of the planned events, statistical significance was met at the interim analysis (hazard ratio [HR] = 0.66; P = .01), and this was followed by similar findings in the primary analysis (HR = 0.73; P = .02), which Dr. Kalinsky presented. At 6 months, the rate of progression-free survival by investigator assessment was 50% vs 37%, respectively (Table 1).
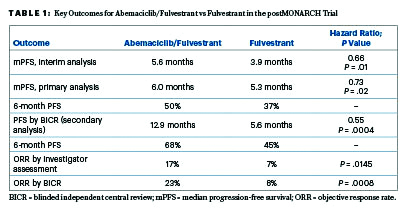
By blinded independent central review, the progression-free survival benefit was even greater, amounting to a 45% reduction in risk. “Of note, there was higher discordance, with more investigator-assessed events as compared to those called by independent central review in the abemaciclib arm compared to the placebo arm. The estimate could be impacted by informative censoring, with likely overestimation of the point estimates,” he explained.
Consistent effects were seen across major clinical and genomic subgroups, including patients with baseline ESR1 or PIK3CA mutations. For patients with measurable disease, the addition of abemaciclib doubled the objective response, whether determined by investigators or blinded central review, Dr. Kalinsky reported.
By prior duration of CDK4/6 inhibitor, the combination was favored in patients with both shorter and longer durations, with the magnitude of benefit higher after at least 12 months of prior treatment. The median progression-free survival for this group was 7.0 vs 5.4 months (HR = 0.70; 95% CI = 0.52–0.94); for those with treatment up to 12 months, it was 5.5 vs 3.0 months (HR = 0.80; 95% CI = 0.50–1.29). Activity was also seen in patients without visceral metastases (HR = 0.53; 95% CI = 0.34–0.83), but “we still saw a benefit in patients with visceral metastases (HR = 0.87; 95% CI = 0.64–1.17),” Dr Kalinsky added.
Safety was consistent with the known profile of abemaciclib. Grade 3 neutropenia was reported in 25% of the doublet arm; 6% of patients on the doublet discontinued treatment because of an adverse event, compared with no patients on fulvestrant alone.
DISCLOSURE: Dr. Kalinsky reported financial relationships with 4D Pharma, AstraZeneca, Cullinan Oncology, Daiichi Sankyo/Astra Zeneca, eFFECTOR Therapeutics, Genentech/Roche, Immunomedics, Lilly, Menarini Silicon Biosystems, Merck, Mersana, Myovant Sciences, Novartis, Oncosec, Prelude Therapeutics, Puma Biotechnology, RayzeBio, Seagen, Takeda, EQRx, and GRAIL.
REFERENCE
1. Kalinsky K, et al: 2024 ASCO Annual Meeting. Abstract LBA1001. Presented June 1, 2024.
EXPERT POINT OF VIEW
The invited discussant of postMONARCH was Ruth O’Regan, MD, Chair of Medicine and Charles A. Dewey Professor at the University of Rochester. “In investigator-assessed progression-free survival,” she commented, “the difference was about 1 month but nonetheless was statistically significant. The greatest benefit was in patients who got prolonged benefit from prior CDK4/6 inhibitor and endocrine therapy in the first-line setting and in patients without visceral metastases.”

Ruth O’Regan, MD
Dr. O’Regan put postMONARCH’s findings into context with three other trials that evaluated continuing CDK inhibition after disease progression—MAINTAIN,1 PALMIRA,2 and PACE.3 They obviously have some important differences, she said, but they are similar in that the most common CDK4/6 inhibitor used in the first-line setting prior to disease progression was palbociclib.
The postMONARCH study showed a 0.7-month improvement by switching to abemaciclib. MAINTAIN achieved a 2.5-month improvement by switching to ribociclib. Both PALMIRA and PACE continued patients on palbociclib and showed no significant improvements: 1.3-month increase and –0.2 month decrement, respectively. Dr. O’Regan further noted that the control arms performed better in postMONARCH compared to MAINTAIN, likely reflecting a more endocrine-sensitive population, since all patients were treated in a purely second-line setting.
Clinical Implications
Dr. O’Regan noted: “Switching CDK4/6 inhibitor at [disease] progression is an option for patients in the second-line or later setting, especially in the absence of a targetable mutation.” For patients with hormone receptor–positive, HER2-negative advanced breast cancer who experience disease progression on CDK4/6 inhibitors, her selection of a second-line treatment depends on the presence or absence of a targetable mutation, since there are approved drugs targeting ESR1 mutations, PI3K mutations, and AKT alterations. In the absence of a mutation, the findings of postMONARCH and MAINTAIN support switching to a different CDK4/6 inhibitor and a different endocrine therapy. Other options for this line include chemotherapy, antibody-drug conjugates, and everolimus plus endocrine therapy.
DISCLOSURE: Dr. O’Regan reported financial relationships with Lilly, Novartis, Pfizer, Puma Biotechnology, and Gilead.
REFERENCES
1. Kalinsky K, et al: J Clin Oncol 41:4004-4013, 2023.
2. Llombart-Cussac A, et al: 2023 ASCO Annual Meeting. Abstract 1001.

