A rapid update to the ASCO guideline on systemic therapy for melanoma adds a new recommendation for the treatment of patients with metastatic uveal melanoma.1 The update follows the January 2022 U.S. Food and Drug Administration (FDA) approval of tebentafusp-tebn for patients with previously untreated HLA-A*02:01–positive metastatic uveal melanoma.2

Pauline Funchain, MD

Rahul Seth, DO
“This is a monumental approval, both clinically and scientifically,” said Pauline Funchain, MD, of the Cleveland Clinic, guideline Expert Panel Co-Chair. “Clinically, tebentafusp is the first FDA-approved drug for metastatic uveal melanoma. Scientifically, tebentafusp is a first-in-class drug in two arenas—not only as the first approved T-cell receptor therapy but [also as] the first bispecific protein.”
Uveal melanoma “has never had a real success story, nor have there been any targeted therapies for metastatic uveal melanoma,” said Rahul Seth, DO, of the SUNY Upstate Medical University, guideline Expert Panel Co-Chair. “[Tebentafusp] is a remarkable drug, for it is the first of its kind … [and] has been able to give patients a fighting chance.”
Tebentafusp consists of an affinity-enhanced T-cell receptor fused to an anti-CD3 effector that recruits T cells to target cells expressing the target antigen, glycoprotein 100. It received FDA approval after demonstrating an overall survival benefit in the phase III IMCgp100-202 trial, with a 1-year overall survival rate of 73% compared with 59% with a control therapy (hazard ratio = 0.51, 95% confidence interval = 0.37–0.71, P < .001).3
Updated Recommendation
The 2022 ASCO melanoma guideline update strongly recommends tebentafusp for patients with previously untreated HLA-A*02:01–positive metastatic uveal melanoma.1 For all other patients with uveal melanoma, there is no recommendation for or against any specific systemic therapy, and clinical trial enrollment should be considered where possible.
IMCgp100-202 was an open-label, randomized trial designed to compare tebentafusp with an investigator’s choice of treatment as first-line systemic therapy for patients with metastatic uveal melanoma.3 A total of 378 patients with HLA-A*02:01–positive disease were randomly assigned 2:1 to intravenous tebentafusp (dosed at 20 mg on day 1, 30 mg on day 8, and 68 mg weekly thereafter) or to investigator’s choice of treatment with single-agent pembrolizumab, ipilimumab, or dacarbazine. After a median follow-up of 14.1 months, tebentafusp was associated with a significant 49% reduction in the risk of death vs control therapy (Table 1).
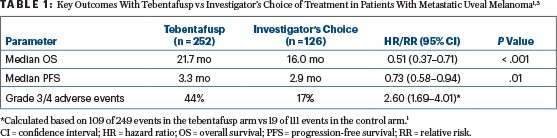
The most common treatment-related adverse events reported in the tebentafusp group were cytokine-related (eg, pyrexia, chills, and hypotension) and skin-related, including rash, pruritus, and erythema.3 The likelihood of developing a grade 3 or 4 treatment-related adverse event was increased by 2.6-fold with tebentafusp vs control therapy (Table 1, page 43).1 Study investigators noted that treatment-related adverse events occurred most frequently in the first 4 weeks of treatment, with the incidence and severity decreasing with repeated dosing. A total of 2% of patients discontinued tebentafusp because of adverse events.3
Dr. Funchain pointed out that the clear improvement in overall survival with tebentafusp was “muddled by a very unimpressive objective response rate” of 9%, compared with 5% in the control arm.3 Unusually, despite the lack of objective responses with tebentafusp, the clear overall survival benefit associated with tebentafusp extended even to patients with progressive disease on treatment.
“I think this dissociation between radiologic response and survival points to a different paradigm of therapeutic response than we are used to seeing with traditional chemotherapeutic and targeted therapies,” she said, suggesting there may be a need to develop different measures of efficacy, and different biomarkers of response for these newer immunotherapy-based drugs.
Looking ahead, Dr. Seth called these types of drugs “the beginning of a new class that may change the overall outcome of melanoma, not just uveal melanoma,” concluding that these developments may lead to better adjuvant therapy as well.
REFERENCES
1. Seth R, Messersmith H, Funchain P, et al: Systemic therapy for melanoma guideline expert panel. J Clin Oncol. June 3, 2022 (early release online).
2. U.S. Food and Drug Administration: FDA approves tebentafusp-tebn for unresectable or metastatic uveal melanoma. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tebentafusp-tebn-unresectable-or-metastatic-uveal-melanoma. Accessed May 16, 2022.
3. Nathan P, Hassel JC, Rutkowski P, et al: Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 385:1196-1206, 2021.
Originally published in ASCO Daily News. © American Society of Clinical Oncology. ASCO Daily News, June 4, 2022. All rights reserved.

