In the final, preplanned, overall survival analysis in the randomized phase III SOLO2/ENGOT-ov211 trial, maintenance treatment with the PARP inhibitor olaparib extended overall survival by an unprecedented 12.9 months, compared with placebo. This marks the first time that overall survival has been improved with maintenance therapy involving a poly (ADP-ribose) polymerase (PARP) inhibitor in patients with platinum-sensitive recurrent ovarian cancer associated with BRCA1/2 mutations.1
Andrés Poveda, MD, of Initia Oncology, Hospital Quirónsalud, Valencia, Spain, announced the results at the Plenary Program of the ASCO20 Virtual Scientific Program. “A long-term treatment benefit was seen with olaparib vs placebo, with an overall survival hazard ratio of 0.74 in the full-analysis set, which was unadjusted for crossover,” Dr. Poveda stated.

Andrés Poveda, MD
“SOLO2 is the first phase III trial to provide final overall survival data on maintenance PARP inhibitor therapy. A median overall survival improvement of nearly 13 months is impressive in ovarian cancer and brings a substantial benefit to our patients,” he commented.
Olaparib is approved as maintenance therapy for patients with platinum-sensitive relapsed ovarian cancer, regardless of BRCA mutation status, in numerous countries.
ASCO Chief Medical Officer and Executive Vice President Richard L. Schilsky, MD, FACP, FSCT, FASCO, commented in the press briefing: “These results, while they will not change access to the drug because it’s already approved, are comforting in showing that the treatment confers a significant survival benefit. That’s good news for women with ovarian cancer harboring BRCA1/2 mutations, which generally has a poor prognosis.”

Richard L. Schilsky, MD, FACP, FSCT, FASCO
SOLO2 Details
The current report is the preplanned, final, overall survival analysis of the study, which was conducted in the germline BRCA-mutated subset and finalized February 3, 2020, with data maturity of 61%. SOLO2 had already shown that maintenance treatment with olaparib significantly improved median progression-free survival by 13.6 months vs placebo (hazard ratio [HR] = 0.30; P < .0001).2 The time to second disease progression or death significantly improved as well, and a quality-adjusted progression-free survival benefit was observed.
The study enrolled 295 patients with relapsed BRCA-related high-grade serous ovarian cancer or high-grade endometrioid cancer, including primary peritoneal or fallopian tube cancer. All had received at least two prior lines of therapy and were in response to their most recent platinum-based regimen. Women were randomly assigned to receive maintenance olaparib (300 mg twice daily; n = 195) or placebo (n = 99), continued until disease progression.
Crossover was noted for 39% of the placebo arm; 11% of the olaparib arm received a subsequent PARP inhibitor. Patients were followed for a median of 65 months.
Survival Benefit Shown
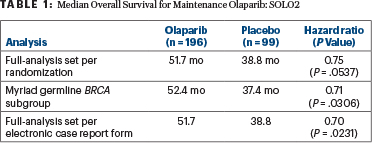
Olaparib extended overall survival, which was a secondary endpoint, by approximately 13 months, compared with placebo, and this was consistent across three analyses: the full-analysis set, which was unadjusted for crossover; the full, prespecified sensitivity analysis of patients with germline BRCA-mutated disease; and the post hoc sensitivity analysis that used stratification variables based on electronic case reports to correct for patients who had been erroneously stratified at randomization (Table 1). At 5 years, 42% of the olaparib arm was alive, compared with 33% of the placebo arm (HR = 0.74; P = .0537), Dr. Poveda reported.
The toxicity was consistent with the known side effects of olaparib. The most common grade ≥ 3 treatment-emergent adverse event was anemia, which led to dose interruptions in 50% of patients (vs 19% with placebo), dose reduction in 28% (vs 3%), and treatment discontinuations in 17% (vs 3%).
DISCLOSURE: This study was funded by AstraZeneca and Merck Sharp & Dohme Corp. Dr. Poveda has consulted or advised for AstraZeneca, Clovis Oncology, PharmaMar, Roche, and Tesaro and has received travel funding from PharmaMar. Dr. Schilsky has received institutional research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech/Roche, Lilly, Merck, and Pfizer and has been reimbursed for travel, accommodations, or other expenses by Varian.
REFERENCES
1. Poveda A, Floquet A, Ledermann JA, et al: Final overall survival results from SOLO2/ENGOT-ov21: A phase III trial assessing maintenance olaparib in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA mutation. ASCO20 Virtual Scientific Program. Abstract 6002.
2. Pujade-Lauraine E, Ledermann JA, Selle F, et al: Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 18:1274-1284, 2017.


