Kamal Menghrajani, MD

Martin S. Tallman, MD
Although several treatment options are available for patients with myelodysplastic syndromes (MDS), hematopoietic stem cell transplantation (HSCT) remains the only curative therapy.1 The risks of complications and death from transplantation can be substantial. Determining which patients may benefit most from transplantation and discovering potential signatures to identify them have become topics of great interest within the field and are the seminal questions behind the work of Lindsley and colleagues, as reviewed in this issue of The ASCO Post.2
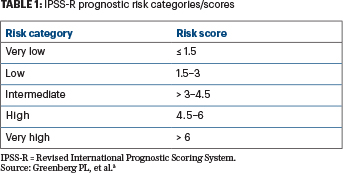
In 2012, Greenberg et al published a revised international prognostic scoring system (IPSS-R) for patients with MDS (Table 1).3 Their system provides clinicians with knowledge about disease prognosis, and it is now routinely used to inform decisions regarding which patients are most likely to experience disease progression, when to initiate treatment, and when to consider stem cell transplantation. The revised IPSS included more cytogenetic subtypes than the original IPSS (first published in 1997); however, this classification did not include molecular genetic information.
The mutational profile of a patient can drastically change prognosis after hematopoietic stem cell transplantation and may lead some patients to choose clinical trials of novel treatment strategies over a high-risk stem cell transplant.— Kamal Menghrajani, MD, and Martin S. Tallman, MD
Tweet this quote
The utility of mutational analysis in understanding the prognosis of MDS became clear in 2013, when Papaemmanuil and colleagues performed targeted gene sequencing on cells from patients with MDS. They found oncogenic mutations in 549 of 738 patients studied, showing that 74% of their cohort had at least one driver mutation, and most had two or more mutations.4 These researchers described the effects of some of these mutations on leukemia-free survival and the impact of mutational burden on the risk of transformation to acute myeloid leukemia (AML), solidifying the notion that molecular analysis should routinely be done in patients with MDS.
In 2014, Bejar and colleagues showed that some of these mutations could also predict poor outcomes after stem cell transplantation. In their series of 87 MDS patients, they found that 92% had oncogenic mutations.5 No mutations were associated with longer progression-free or overall survival, and TP53 mutations were associated with significantly shortened durations of both progression-free survival and overall survival.
TP53 Mutations Predict Early Relapse and Mortality
Expanding on their previous work, Lindsley, Ebert, and colleagues have since performed a targeted mutational analysis on a cohort of 1,514 patients with MDS who were registered with the Center for International Blood and Marrow Transplant Research (CIBMTR). The investigators examined associations between specific mutations and overall survival, relapse, and death without relapse.2 They sequenced 129 genes, compared to 40 in their group’s 2014 publication, and found at least 1 mutation in 79% of patients; they then evaluated the prognostic significance of the 32 genes that were mutated in at least 20 patients.
They discovered that mutated TP53 was, once again, a high-risk feature. A mutation in this gene was found in 19% of patients and was a harbinger of both shorter time to relapse and shorter overall survival. These investigators evaluated whether the conditioning regimen used for transplantation influenced outcome and observed that both reduced-intensity and myeloablative conditioning regimens carried the same risk of relapse and death in patients with TP53 mutation. Not surprisingly, truncating mutations that were likely to lead to a loss of TP53 activity appeared to be more deleterious, resulting in shorter survival, than TP53 missense mutations. This study also confirmed the previous observation that TP53 mutations are more commonly seen in therapy-related MDS.6
Lindsley and coworkers also reported that mutations in the RAS pathway among patients older than 40 years were associated with a shorter time to relapse, but only in those treated with reduced-intensity as opposed to myeloablative conditioning. JAK2 mutations correlated with decreased survival but, interestingly, not with an increased risk of relapse; this association was seen with both types of conditioning regimens.
Mutational Analysis Can Influence Treatment Decisions
This study confirms how essential the use of molecular testing has become in the understanding of MDS. While mutated TP53 was previously described as a high-risk feature, Lindsley and colleagues show that increased intensity of conditioning prior to stem cell transplantation does not modify this risk. As such, it may be worthwhile to study whether reduced-intensity conditioning should be considered the standard regimen for all patients with TP53-mutated MDS who undergo transplantation.
Additionally, patients with MDS who have particular mutations are in need of novel therapeutic strategies. Patients with JAK2 mutations may benefit from single-agent or combination-therapy trials using JAK2 inhibitors, which could improve their overall survival. Further evaluation of the mechanisms by which the presence of a JAK2 mutation leads to shorter overall survival—in the absence of an increased risk of relapse—may help inform these treatment decisions.
Patients over 40 years old with RAS pathway mutations may benefit from the exclusive use of myeloablative conditioning regimens, as a way to minimize their risk of relapse after stem cell transplantation. Additionally, patients with TP53 mutations may benefit from alternative strategies before transplantation. A 10-day course of treatment with the hypomethylating agent decitabine was described in a retrospective study to result in responses among a cohort of 21 MDS and AML patients, but it is not yet clear whether this approach delays the need for transplantation or modifies the disease course after a transplant has been performed.7
Finally, new prognostic models should take into account molecular testing, which is rapidly becoming standard of care. The study discussed here clearly demonstrates that the mutational profile of a patient can drastically change prognosis after hematopoietic stem cell transplantation and may lead some patients to choose clinical trials of novel treatment strategies over a high-risk stem cell transplant. ■
Dr. Menghrajani is a second-year fellow in the Department of Medicine, Memorial Sloan Kettering Cancer Center. Dr. Tallman is Chief of the Leukemia Service, Memorial Sloan Kettering Cancer Center, and Professor of Medicine, Weill Cornell Medical College, New York.
Disclosure: Drs. Menghrajana and Tallman reported no conflicts of interest.
References
1. Greenberg PL, Stone RM, Al-Kali A, et al: Myelodysplastic syndromes, version 2.2017. NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15:60-87, 2017.
2. Lindsley RC, Saber W, Mar BG, et al: Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med 376:536-547, 2017.
3. Greenberg PL, Tuechler H, Schanz J, et al: Revised International Prognostic Scoring System for myelodysplastic syndromes. Blood 120:2454-2465, 2012.
4. Papaemmanuil E, Gerstung M, Malcovati L, et al: Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122:3616-3627, 2013.
5. Bejar R, Stevenson KE, Caughey B, et al: Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol 32:2691-2698, 2014.
6. Ok CY, Patel KP, Garcia-Manero G, et al: Mutational profiling of therapy-related myelodysplastic syndromes and acute myeloid leukemia by next generation sequencing, a comparison with de novo diseases. Leuk Res 39:348-354, 2015.
7. Welch JS, Petti AA, Miller CA, et al: TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med 375:2023-2036, 2016.

