At the 2015 ASCO Annual Meeting, Annick Desjardins, MD, Associate Professor of Neurology at Duke University Medical Center, presented a brief update on the ongoing study of oncolytic PVS-RIPO in glioblastoma, which now includes 24 patients.1 The median age of enrolled patients is 57, most have a good performance status, all received radiation therapy and temozolomide, and 42% also received bevacizumab (Avastin).
As of May 19, 2015, 11 patients had died. Median survival was 12.5 months, the 6-month survival rate was 82.7%, and the 12-month survival rate was 56%, Dr. Desjardins reported.
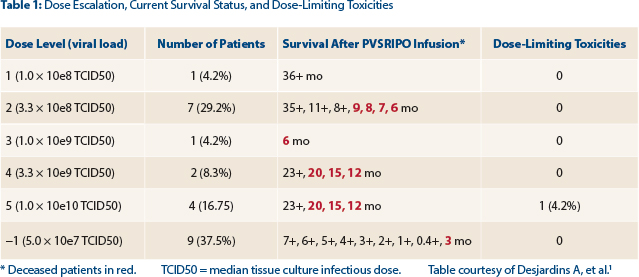
Of the 24 patients, 9 have been treated at the optimal dose level, and they are anticipated to have longer survival. Only one has reached the 6-month mark, however, so “it’s too early to tell,” she said.
The survival data for all patients at all dose levels are shown in Table 1. The study continues to enroll at “dose level minus 1” (−1).
Adverse Events
Most adverse events have been related to secondary inflammatory response and prolonged steroid use, she said. Grade ≥ 3 neurologic adverse events have included hemiparesis in six patients (14.3%) and steroid myopathy, intracranial hemorrhage upon catheter removal, and seizure in one patient each. Grade ≥ 3 hematologic toxicity has included lymphopenia in six patients. Other toxicities include hyperglycemia in three patients and hypophosphatemia, fatigue, pneumonia, skin infection, and a thromboembolic event in one patient each.
“At dose level −1, we are seeing many fewer inflammation issues (such as seizures and weakness) than we saw in other dose cohorts, and efficacy is just as good,” Dr. Desjardins said.
The investigators started bevacizumab in patients who appear to require more than 4 mg/d of dexamethasone to control inflammation. They used 7.5 mg/kg of bevacizumab every 3 weeks, which is much lower than the therapeutic dose. ■
Disclosure: Dr. Desjardins is a co-owner of the intellectual property related to the technology discussed, for which a patent is pending.
Reference
1. Desjardins A, Sampson JH, Peters KB, et al: Oncolytic polio/rhinovirus recombinant (PVSRIPO) against recurrent glioblastoma: Optimal dose determination. 2015 ASCO Annual Meeting. Abstract 2068. Presented June 1, 2015.