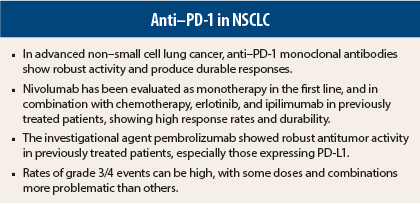
Monoclonal antibodies targeting the programmed death receptor-1 (PD-1) pathway are expected to answer an unmet need in non–small cell lung cancer (NSCLC). With first-line platinum-doublets, 1-year overall survival is 30% to 50%, and while treatments targeting sensitizing mutations are more effective, they benefit only a few subsets of patients.
Data presented at the 2014 ASCO Annual Meeting point to the anti–PD-1 agents as a means of filling this treatment gap.
Underlying Mechanism
PD-1 is an inhibitory T-cell co-receptor expressed by activated T cells, whose pathway is hijacked by tumors to suppress immune control. PD-1 interacts with its ligands, PD-L1 and PD-L2, and this activation can lead to suppression of antitumor immunity. Anti–PD-1 agents bind with the PD-1 receptor on T cells, selectively blocking its interaction with its ligands. This restores T-cell antitumor function.
Very promising results were reported for nivolumab in the phase I CA209-012 multicohort study, which evaluated single-agent nivolumab, nivolumab combined with ipilimumab (Yervoy), and nivolumab combined with erlotinib (Tarceva) in the first-line setting, as well for the newest agent, pembrolizumab (formerly known as lambrolizumab).
Pembrolizumab in Previously Treated Patients
In a phase I study of 217 previously treated NSCLC patients, pembrolizumab at 10 mg/kg every 2 weeks or 3 weeks demonstrated “robust antitumor activity,” especially among patients whose tumors expressing PD-L1, according to Edward B. Garon, MD, Assistant Professor of Medicine at the University of California, Los Angeles.1
The objective response rate was 20%, and was higher among the 159 patients with tumors expressing PD-L1 (23%) than among those who did not (9%). Of the PD-L1–positive patients who responded, 82% remain on treatment. Compared with baseline, 58% of PD-L1–positive patients vs 30% of the negative group had some reduction in tumor size by RECIST criteria.
Nearly two-thirds of patients had at least one drug-related adverse event of any grade, and 10% had grade 3/4 toxicity. Grade 3/4 pneumonitis was observed in four patients.
Single-Agent Nivolumab in First Line
Scott N. Gettinger, MD, Associate Professor of Medicine at Yale Cancer Center, New Haven, presented interim data for nivolumab at 3 mg/kg every 2 weeks in chemotherapy-naive patients.2 The objective response rate was 30% and median duration of response had not been reached. Two patients had complete responses, with another unconfirmed, whereas 35% had stable disease, most lasting at least 24 weeks.
Progression-free survival rate at 24 weeks was 60%, median progression-free survival was 47.2 weeks in patients with nonsquamous histology and 15.1 in those with squamous tumors, and 1-year overall survival rate was 75%. Median overall survival was not reached at the time of analysis.
All the responders were found to have PD-L1 staining in at least 5% of tumor cells (ie, were PD-L1–positive). In these patients, progression-free survival rate at 24 weeks was 70%, compared to 57% in PD-L1–negative patients. Median progression-free survival was 45.6 weeks and 36.1 weeks, and 1-year overall survival rate was 80% and 71%, respectively.
Treatment-related adverse events were observed in 85% of patients, mostly grade 1/2. Grade 3/4 toxicity was noted in only 20%.
“First-line treatment for advanced NSCLC with nivolumab monotherapy demonstrated early and durable responses, and encouraging progression-free and overall survival profiles,” Dr. Gettinger concluded. “These interim results support further studies of first-line nivolumab monotherapy and continued evaluation of PD-L1 expression as a potential biomarker for activity.”
Nivolumab Plus Ipilimumab
There is great interest in dual immune checkpoint blockade, based on robust efficacy for the combination in advanced melanoma. Results from another arm of the phase I CA209-012 study, presented by Scott J. Antonia, MD, PhD, Chair of Thoracic Oncology at H. Lee Moffitt Cancer Center, Tampa, Florida, were promising.3
A total of 49 chemotherapy-naive patients received nivolumab plus ipilimumab every 3 weeks for four cycles, followed by nivolumab monotherapy every 2 weeks until progression. Two schedules were evaluated: nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg or nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg.
Depending on dose and histology, objective responses were observed in 11% to 33% of patients, the highest (33%) seen in squamous disease patients receiving nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg. Responses were ongoing in 75% of responders at the time of analysis. Overall, 60% of evaluable patients experienced a decrease in tumor burden.
Median progression-free survival at 24 weeks ranged from 20% to 51% and median progression-free survival was 14.4 to 16.1 weeks. Median overall survival was reached only in patients of squamous histology receiving nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg 16.1 weeks (44.3 weeks), and was not reached in the other arms.
Exploratory PD-L1 analysis revealed that response rates and median progression-free survival were similar whether patients were PD-L1–positive or –negative, and median overall survival was not reached in either group.
“The findings suggest this combination may be suitable for PD-L1−positive and −negative patients,” Dr. Antonia concluded. “There was no apparent correlation between response rate, progression-free survival, and overall survival and PD-L1 status, similar to previous observations with combined nivolumab plus ipilimumab in melanoma.”
Toxicity occurred mainly during induction. Grade 3/4 adverse events were reported by 36% to 54% of patients across the combination doses during induction, and by 17% to 21% in the maintenance phase. Grade 3/4 toxicities were reported by 49% across the arms. Grade 3/4 pneumonitis occurred in 6% and was reversible. Serious diarrhea and colitis occurred in 8%, and increased liver enzymes in 6%. One-third of patients discontinued due to adverse events, mostly those receiving nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg, and mostly during concurrent dosing.
Three patients died due to drug-related toxicities, one each from colitis, pulmonary hemorrhage, and toxic epidermal necrolysis (the patient had a history of ulcerative colitis).
Nivolumab Plus Chemotherapy
Dr. Antonia also presented results on the 56 patients who received nivolumab at 10 mg/kg every 3 weeks plus a platinum-based doublet (gemcitabine/cisplatin, pemetrexed (Alimta)/cisplatin, or paclitaxel/carboplatin), followed by nivolumab at 5 mg/kg or 10 mg/kg until progression.4
Objective responses were observed in 33% to 47% of patients, and in both squamous and nonsquamous histology subsets. A decrease in tumor burden was noted in 84%.
Outcomes were impressive in the subgroup receiving pemetrexed/cisplatin plus nivolumab at 10 mg/kg. Their 24-week progression-free survival rate was 71% (vs 36% to 57% of the other arms); 1-year overall survival was 87%, and median overall survival was 83.4 weeks, Dr. Antonia reported.
With paclitaxel/carboplatin plus nivolumab at 5 mg/kg, median overall survival was not reached, and median progression-free survival was 31 weeks.
Treatment-related grade 3/4 adverse events occurred in 45%, most commonly pneumonitis, fatigue, and acute renal failure. Most (73%) were seen with nivolumab at 10 mg/kg plus paclitaxel/carboplatin. “Many of these were laboratory abnormalities reported in one patient each,” he explained.
“The safety profile reflects the additive toxicity of nivolumab and chemotherapy,” he said. Discontinuations due to drug-related events were observed mainly during maintenance.
Nivolumab Plus Erlotinib
Naiyer A. Rizvi, MD, medical oncologist at Memorial Sloan Kettering Cancer Center, New York, presented the CA209-012 data on nivolumab combined with erlotinib in patients with epidermal growth factor receptor (EGFR) mutations.5
“Recent data suggest that constitutive oncogenic signaling through the EGFR pathway may promote tumor immune escape by inducing immune dysfunction in the tumor microenvironment,” he explained. Treatment of EGFR-mutant NSCLC cell lines with an EGFR tyrosine kinase inhibitor downregulates PD-L1 expression.
The study included 21 patients with EGFR-mutated adenocarcinoma and prior erlotinib treatment; 33% had the deleterious T790M mutation. Of 21 patients, 4 (19%) responded, and responses were ongoing in 3 patients at the time of analysis. The estimated duration of response was 60 weeks, and 15% of patients had stable disease.
“Responses were seen in patients who had previously received multiple lines of EGFR inhibitor therapy, as well as patients with T790M mutations,” Dr. Rizvi reported.
Progression-free survival at 24 weeks was 51%, median progression-free survival was 29.4 weeks, 1-year survival was 73%, and median overall survival was not yet reached. The toxicities were additive, but only 24% were grade 3/4.
The data suggest that “combining PD-1 blockade with therapies that target mutant EGFR signaling may be a novel approach to enhancing the treatment response, and may provide durable benefit. The response rate and duration of response are encouraging, relative to other available therapies for such patients,” he concluded. ■
Disclosure: Dr. Garon is an unpaid consultant/advisor for and has received research funding from Merck. Dr. Gettinger is a consultant/advisor for, has stock ownership in, and has received honoraria from Bristol-Myers Squibb. Dr. Antonia is a consultant/advisor for, has stock ownership in, and has received honoraria from Bristol-Myers Squibb, and has received research funding from MedImmune. Dr. Rizvi is a consultant/advisor for Bristol-Myers Squibb, has received honoraria from Bristol-Myers Squibb, MedImmune, and Roche/Genentech, and has received research funding from Arisaph Pharmaceuticals, Millennium, and Pfizer.
References
1. Garon EB, Leighl NB, Rizvi NA, et al: Safety and clinical activity of MK-3475 in previously treated patients with non-small cell lung cancer. ASCO Annual Meeting. Abstract 8020. Presented June 3, 2014.
2. Gettinger SN, Shepherd FA, Antonia SJ, et al: First-line nivolumab monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. ASCO Annual Meeting. Abstract 8024. Presented June 3, 2014.
3. Antonia SJ, Gettinger SN, Chow LQ, et al: Nivolumab and ipilimumab in first-line NSCLC: Interim phase 1 results. ASCO Annual Meeting. Abstract 8023. Presented June 3, 2014.
4. Antonia SJ, Brahmer JR, Gettinger SN, et al: Nivolumab in combination with platinum-based doublet chemotherapy in advanced non-small cell lung cancer. ASCO Annual Meeting. Abstract 8113. Presented May 31, 2014.
5. Rizvi NA, Chow LQ, Borghael H, et al: Safety and response with nivolumab plus erlotinib in patient with epidermal growth factor receptor mutant advanced NSCLC. ASCO Annual Meeting. Abstract 8022. Presented June 3, 2014.