The combination of lenalidomide (Revlimid) and rituximab (Rituximab), dubbed the “R-squared” regimen, has gained attention lately, and ongoing trials are evaluating whether chemotherapy with R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, prednisone) can be improved by adding a nonchemotherapeutic agent like lenalidomide. At the 2014 ASCO Annual Meeting, researchers reported encouraging results of this approach in diffuse large B-cell lymphoma, especially with the non–germinal center B-cell (non-GCB) phenotype.1
Phase II Study
In a phase II study of 64 patients with newly diagnosed diffuse large B-cell lymphoma, the addition of lenalidomide to R-CHOP (R2-CHOP), appeared to reduce the negative prognostic significance of the non–germinal center B-cell phenotype, producing progression-free and overall survival rates similar to those observed in the germinal center B-cell subtype, reported Grzegorz S. Nowakowski, MD, Assistant Professor of Medicine at the Mayo Clinic, Rochester, Minnesota.
“The addition of lenalidomide may ameliorate the negative effect of [non–germinal center B-cell] phenotype on outcome,” Dr. Nowakowski said.
The R2-CHOP treatment consisted of 25 mg lenalidomide daily on days 1 to 10 of a 21-day cycle in addition to standard R-CHOP given every three cycles. The population had a median age of 65, with 20% older than 70 years. Sixty percent had stage IV disease, and about half the patients were considered high-risk.
Key Findings
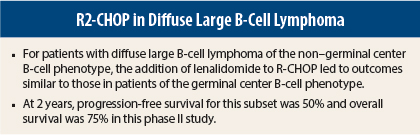
Among 55 evaluable patients, 33 were of the germinal center B-cell phenotype and 22 were non–germinal center B-cell patients. The R2-CHOP regimen was associated with similar outcomes in both subtypes, with 2-year progression-free survival of 60% and 50%, respectively, and 2-year survival rates of 83% and 75%, Dr. Nowakowski said.
The response rate was 98%, and 80% of patients had complete responses.
The R2-CHOP regimen was well tolerated, even in the older patients, he said. The most frequent grade 3/4 toxicity was neutropenia (87%), but febrile neutropenia was rare. Grade 3/4 thrombocytopenia (44%) and anemia (15%) were observed in some patients.
A case-matched historical analysis showed that outcomes with R-CHOP were substantially worse among patients with non–germinal center B-cell phenotype than for those with germinal center B-cell diffuse large B-cell lymphoma. At 2 years, 64% of germinal center B-cell patients were progression-free, compared to only 28% of the non–germinal center B-cell population treated with R-CHOP (P = .00029). However, in the R2-CHOP treatment arms, these rates were 59% and 60%, respectively (P = .83).
Similarly, 2-year overall survival was 74% and 46%, respectively, in the R-CHOP treatment arms (P = .000036), but were comparable in the R2-CHOP arms—75% and 83%, respectively
(P = .61).
These findings suggest that the addition of lenalidomide to R-CHOP may overcome the negative prognostic impact associated with the non–germinal center B-cell phenotype, he suggested.
A randomized trial, Eastern Cooperative Oncology Group (ECOG) 1412, is currently comparing R2-CHOP vs R-CHOP for the initial treatment of diffuse large B-cell lymphoma. The study is also enrolling patients through the Alliance and SWOG. ■
Disclosure: Dr. Nowakowski and his coauthors reported no potential conflicts of interest.
Reference
1. Nowakowski GS, LaPlant B, Macon WR, et al: Effect of lenalidomide combined with R-CHOP (R2CHOP) on negative prognostic impact of nongerminal center phenotype in newly diagnosed diffuse large B-cell lymphoma: A phase 2 study. ASCO Annual Meeting. Abstract 8520. Presented June 1, 2014.