As a neoadjuvant regimen for HER2-positive early breast cancer, the use of two HER2-directed agents was no more effective than trastuzumab (Herceptin) alone in producing pathologic complete responses, although one subset of patients did benefit from this approach, according to the results of the Cancer and Leukemia Group B (CALGB) 40601 trial reported at the 2013 ASCO Annual Meeting by Lisa A. Carey, MD, Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research at The University of North Carolina at Chapel Hill.1
“The addition of lapatinib [Tykerb] to 16 weeks of weekly trastuzumab/paclitaxel did not meet the predefined criteria for a significant increase in pathologic complete responses,” she announced at the Annual Meeting. “The numerical increase is modest. We now need to determine which tumors can benefit from treatment with dual vs single HER2 targeting.”
The study did show higher pathologic complete response rates, however, among the molecular subtype defined as HER2-enriched.
CALGB 40601 questioned whether patients with stage II or III HER2-positive breast cancer could benefit more from two anti-HER2 agents vs single-agent trastuzumab, plus chemotherapy in the adjuvant setting.
“Early evidence in stage IV disease and in the neoadjuvant setting have suggested that dual targeting may be beneficial, using mainly lapatinib or pertuzumab [plus trastuzumab],” she said. “There are adjuvant trials in progress evaluating dual targeting (ALTTO, Aphinity), so this question is quite relevant.”
Patients were randomly assigned to receive trastuzumab/paclitaxel, trastuzumab/paclitaxel plus lapatinib, or lapatinib/paclitaxel, all given for 16 weeks prior to surgery. (The third arm was discontinued early due to lack of efficacy and is not part of this analysis.)
Intravenous paclitaxel 80 mg/m2 was given once weekly while lapatinib was given orally at a daily dose of 750 mg. Trastuzumab was given intravenously at a loading dose of 4 mg/kg and then at 2 mg/kg once a week. The primary endpoint was pathologic complete response, defined as the absence of invasive cancer in the breast.
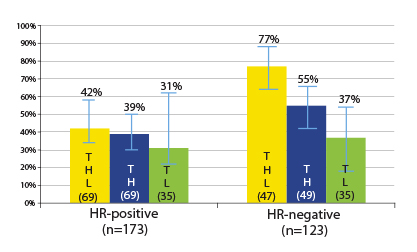
Response Rates Differ by Hormone Receptor Status
The rates of pathologic complete response in the breast were 56% for the three-drug combination and 46% for trastuzumab/paclitaxel, but this 10% difference was not statistically significant.
By hormone receptor status, some differences did emerge. Patients with hormone receptor–negative tumors had higher pathologic complete response rates after dual blockade (77% vs 55%), but the differences were modest in the hormone receptor–positive tumors (42% vs 39%). In either group, the improvement with the addition of lapatinib was not significant (Fig. 1).
When the definition of pathologic complete response incorporated the axillae, results were similar to what was seen in the breast alone, with pathologic complete response rates of 52% with dual targeting and 43% with the single agent in the overall population. Rates in the hormone receptor–
positive group were 42% vs 38%, and in the hormone receptor–negative group were 66% vs 51%, respectively, she said.
Grade 3 and 4 toxicities were higher with the addition of lapatinib.
Dr. Carey noted that other neoadjuvant studies of HER2 inhibition have shown numerical differences with the addition of a second anti-HER2 agent, and two were statistically significant: NeoSPHERE (pertuzumab plus trastuzumab and docetaxel) and NeoALTTO (lapatinib plus trastuzumab and weekly paclitaxel).
“What jumps out is that pathologic complete response is numerically increased with dual targeting, and while this was significant in NeoSPHERE and NeoALTTO, the differences were not significant in our study,” she said, suggesting that underlying differences in study populations might explain this discordance.
She added that the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-41 trial also failed to show significant differences in pathologic complete response. “It is not clear why this is so,” she told The ASCO Post. “However, CALGB 40601 and NSABP B-41 included higher proportions of women with hormone receptor–positive disease.”
HER-enriched Subtype
An exploratory analysis of tissue samples showed that “within the HER2-positive population there is considerable molecular heterogeneity,” Dr. Carey noted. “The intrinsic subtypes appear to differ in sensitivity to HER2-targeting agents, with numerically highest pathologic complete response rates among the HER2-enriched subtype.”
The pathologic complete response rates were 75% among the HER2-enriched subset, but lower for the normal-like subtype (50%), basal-like subtype (36%), and luminal A subtype (35%), and lowest of all for the luminal B subtype (29%).
By treatment arm and subtype, the highest rates were achieved by HER2-enriched patients receiving trastuzumab/paclitaxel plus lapatinib (89%), followed by HER2-enriched patients receiving trastuzumab/paclitaxel alone (80%). The rates were lowest (25%) for patients with basal-like and luminal B tumors receiving only trastuzumab.
Breast-conserving Surgery
A preplanned surgical endpoint was the incidence of breast-conserving therapy, reported by David W. Ollila, MD, Professor of Surgery at The University of North Carolina.2
In the combined treatment arms, 43% of women considered ineligible for breast-conserving surgery at baseline were able to undergo breast-conserving surgery after neoadjuvant therapy. The pathologic complete response rate in this group was 43%; in patients who remained ineligible, 44% achieved a pathologic complete response.
Of the 42% of women initially deemed eligible, 94% ultimately underwent lumpectomy; 55% of this group achieved a pathologic complete response, compared with 29% of women initially eligible but deemed ineligible after neoadjuvant therapy.
Altogether, 76% of all patients were eligible for an attempt at breast-conserving therapy after neoadjuvant therapy, and 79% of these attempts were successful, Dr. Ollila reported.
“Neoadjuvant chemotherapy combined with targeted anti-HER2 treatment permits the potential for breast-conserving therapy in approximately 80% of selected patients,” he said. ■
Disclosure: Dr. Carey has received research funding from Genentech, Sanofi Oncology, and GlaxoSmithKline. Dr. Ollila reported no potential conflicts of interest.
References
1. Carey LA, Berry DA, Ollila D, et al: Clinical and translational results of CALGB 40601. 2013 ASCO Annual Meeting. Abstract 500. Presented June 2, 2013.
2. Ollila DW, Berry DA, Cirrincione C, et al: Impact of neoadjuvant chemotherapy plus HER2-targeting on breast conservation rates. 2013 ASCO Annual Meeting. Abstract 501. Presented June 2, 2013.