In patients with advanced HER2-positive gastroesophageal adenocarcinoma, treatment with the bispecific antibody zanidatamab-hrii and chemotherapy, with or without the PD-1 inhibitor tislelizumab-jsgr, reduced the risk of disease progression or death by 35% over trastuzumab plus chemotherapy in the phase III HERIZON-GEA-01 trial presented as a late-breaker at the 2026 ASCO Gastrointestinal Cancers Symposium.1
“This is the first phase III study in advanced gastroesophageal adenocarcinoma to demonstrate a median progression-free survival that is more than 1 year and a median overall survival of more than 2 years,” said Elena Elimova, MD, of Princess Margaret Cancer Centre in Toronto, Canada.The study is also the first to show a benefit for a novel HER2-targeted therapy compared to trastuzumab as part of a combination regimen in the first-line setting, she added.

Elena Elimova, MD
Compared to treatment with trastuzumab plus chemotherapy, patients receiving a zanidatamab-containing regimen experienced an absolute 4-month improvement in progression-free survival and a 7-month improvement in overall survival, she noted.
At a press briefing, Dr. Elimova commented that the findings indicate zanidatamab is the preferred HER2-targeted agent, over trastuzumab, for HER2-positive disease. As for the PD-L1 agent, based on the findings she said she would “feel absolutely comfortable” giving tislelizumab.
Background and KEYNOTE-811
As Dr. Elimova pointed out, approximately 20% of patients with gastroesophageal adenocarcinoma (including cancers of the stomach, gastroesophageal junction, and esophagus) have tumors that are HER2-positive and need novel HER2-directed strategies to improve outcomes. With current therapies, outcomes in this population remain “modest,” she said, with a median progression-free survival of around 10 months and a median overall survival of around 20 months. For more than a decade, the standard front-line treatment for HER2-positive metastatic gastroesophageal adenocarcinoma has been trastuzumab plus chemotherapy. For patients whose tumors are PD-L1–positive, pembrolizumab is now a standard component, based on results of KEYNOTE-811.2 Relapse within 1 year is still common, however.
Zanidatamab, which is approved in metastatic biliary tract cancer, is a dual HER2-targeted bispecific antibody that binds to two distinct sites on HER2. This binding leads to the crosslinking of neighboring HER2 proteins and receptor clustering on the cell surface. Its multiple mechanisms of action include enhanced HER2 internalization, reduced downstream signaling, and immune-mediated cytotoxicity. Tislelizumab, which is approved in PD-L1–positive metastatic gastric and gastroesophageal junction cancer, is a high-affinity immune checkpoint inhibitor targeting PD-1.
About HERIZON-GEA-01
The study enrolled 914 patients with unresectable, locally advanced, recurrent or metastatic gastroesophageal adenocarcinoma; more than two-thirds had gastric cancer. They had no prior treatment in this setting and no prior HER2-targeted therapy or immunotherapy in any setting. They were randomly assigned 1:1:1 to receive trastuzumab plus chemotherapy (Arm A); zanidatamab every 3 weeks plus chemotherapy (Arm B); or zanidatamab every 3 weeks plus tislelizumab every 3 weeks plus chemotherapy (Arm C). CAPOX (capecitabine, oxaliplatin) was the chemotherapy choice for 90% of patients.The dual primary endpoints were progression-free survival by blinded independent review and overall survival.
Key Findings
In this interim analysis, both zanidatamab-containing regimens led to clinically meaningful and statistically significant prolongation of progression-free survival vs trastuzumab plus chemotherapy, yielding more than 4 additional months of remission (Table). The benefits were generally observed across key subgroups, including geographic region and PD-L1 status.
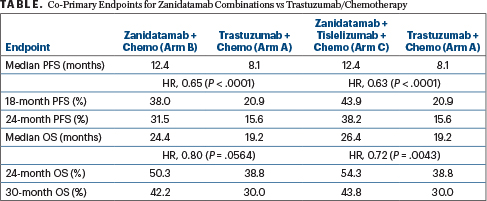
HR = hazard ratio; PFS = progression-free survival; OS = overall survival.
Zanidatamab plus tislelizumab and chemotherapy also demonstrated a statistically significant and clinically meaningful overall survival benefit, providing 7 months longer median overall survival, while zanidatamab plus chemotherapy showed a trend (Table). “At this interim analysis, overall survival data were immature for zanidatamab plus chemotherapy, but there was a strong trend favoring it, with a 5-month longer median overall survival seen,” said Dr. Elimova. The trial is ongoing, and additional overall survival analyses are planned for this arm.
Responses were found to be deeper and more durable in the zanidatamab-containing arms as well. Confirmed objective response rates and complete response rates were 69.6% and 17.1%, respectively, with zanidatamab plus chemotherapy; 70.7% and 19.6%, respectively, with zanidatamab plus tislelizumab plus chemotherapy; and 65.7% and 11.0%, respectively, with trastuzumab plus chemotherapy.Median duration of response was 14.3, 20.7, and 8.3 months, respectively.
While the outcomes were more striking with the zanidatamab plus tislelizumab and chemotherapy regimen, Dr. Elimova reminded journalists at the press briefing that PD-L1 positivity is a requirement for treatment with an immune checkpoint inhibitor. It is likely, therefore, that a subset of patients will be candidates for zanidatamab and chemotherapy alone.
Safety Profile
The safety profile was consistent with the known safety profile of each individual agent. Grade ≥ 3 treatment-related adverse events were reported for 59.0% of the zanidatamab plus chemotherapy arm, 71.8% of the zanidatamab plus tislelizumab plus chemotherapy arm, and 59.6% of the trastuzumab plus chemotherapy arm. Discontinuations due to treatment-related toxicities were noted for 34.4%, 42.5%, and 29.1%, respectively.
Diarrhea was the most common treatment-related toxicity in all three arms. It generally occurred early in treatment and resolved within 3 weeks, and discontinuation of HER2-targeted therapy due to this was infrequent.
DISCLOSURE: Dr. Elimova had personal financial disclosures for Merck, Daiichi Sankyo/AstraZeneca, Roche Canada, AbbVie, Astellas Pharma, BeOne, Signatera, and Viracta Therapeutics.
References
1. Elimova E, Rha SY, Shitara K, et al. Zanidatamab + chemotherapy ± tislelizumab for first-line HER2-positive locally advanced, unresectable, or metastatic gastroesophageal adenocarcinoma: Primary analysis from HERIZON-GEA-01. 2026 ASCO Gastrointestinal Cancers Symposium. Abstract LBA285. Presented January 8, 2026.
2. Janjigian YY, Kawazoe A, Bai Y, et al: Final overall survival for the phase III, KEYNOTE-811 study of pembrolizumab plus trastuzumab and chemotherapy for HER2+ advanced, unresectable or metastatic G/GEJ adenocarcinoma. ESMO Congress 2024. Abstract 1400O. Presented September 14, 2024.
EXPERT POINT OF VIEW
ASCO Expert Rachna Shroff, MD, MS, FASCO, Associate Director of Clinical Investigations and co-leader of the Gastrointestinal Clinical Research Team at the University of Arizona Cancer Center, shared her thoughts on the findings of the phase III HERIZON-GEA-01 trial.

Rachna Shroff, MD, MS, FASCO
“What I think is remarkable about this study is to see a duration of response that is truly meaningful: 20 months with zanidatamab and chemotherapy, in addition to a positive benefit in progression-free survival and at least a trend toward an overall survival benefit with just zanidatamab and chemotherapy,” Dr. Shroff said.
“The findings have the potential to be practice changing,” Dr. Shroff said.
Context: KEYNOTE-811
Current NCCN Guidelines recommend chemotherapy plus trastuzumab alone or with pembrolizumab in PD-L1–positive patients, a regimen approved by the U.S. Food and Drug Administration in March 2025 based on the KEYNOTE-811 data.1“What seems clear is that zanidatamab can, and should, be a new HER2-targeting agent for upper GI cancers.”
According to Dr. Shroff, the most impressive results in HERIZON-GEA-01 were observed when the immune checkpoint agent tislelizumab was added to the regimen. “Keep in mind that this study [HERIZON-GEA-01] was designed before the KEYNOTE-811 data were available, so the arm of zanidatamab plus tislelizumab and chemotherapy (Arm C) is obviously very relevant as we think about adding immunotherapy to HER2-targeting” in patients whose tumors are PD-L1–positive.
For context, in KEYNOTE-811, at a median follow-up of 50.2 months, pembrolizumab added to trastuzumab and chemotherapy in the first-line setting led to a median overall survival of 20.0 vs 16.8 months for placebo plus trastuzumab and chemotherapy (hazard ratio, 0.80; P = .0040). The 36-month overall survival rate was 28% with pembrolizumab and 23% with placebo.
Dr. Shroff added, “Cross-trial comparisons are difficult but the median overall survival from HERIZON with the zanidatamab/tislelizumab/chemotherapy combination was over 2 years. While we will need to see longer-term follow-up and better understand the impact based on PD-L1 status, this combination looks promising. Given the overall survival benefit now seen with chemotherapy plus zanidatamab plus tislelizumab, this combination could likely become the new standard of care, with the recognition that it has not been compared head-to-head with the KEYNOTE-811 combination.”
DISCLOSURE: Dr. Shroff had personal financial relationships with AstraZeneca, Boehringer Ingelheim, Boston Scientific, Genentech, Ipsen, Merus, and Regeneron.
REFERENCE
1. Janjigian YY, Kawazoe A, Bai Y, et al: Final overall survival for the phase III, KEYNOTE-811 study. ESMO Congress 2024. Abstract 1400O. Presented September 14, 2024.

