The oral selective estrogen receptor degrader (SERD) camizestrant improved progression-free survival, as compared with fulvestrant, in previously treated postmenopausal women with advanced breast cancer in the phase II SERENA-2 trial. These results were reported at the 2022 San Antonio Breast Cancer Symposium by Mafalda Oliveira, MD, PhD, a medical oncologist at the Vall d’Hebron University Hospital, Barcelona.1

Mafalda Oliveira, MD, PhD
“In the overall population, at both doses, camizestrant produced a statistically significant and clinically meaningful improvement in progression-free survival over fulvestrant,” Dr. Oliveira said. Benefit was observed in patients with visceral metastases, those with a mutation in the ESR1 gene detected by circulating tumor DNA (ctDNA) analysis, and those who have had prior treatment with a cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor.
Targeting ESR1 Mutation
As Dr. Oliveira pointed out, most hormone receptor–positive, HER2-negative cancers eventually develop resistance to available endocrine therapies. A common mechanism for this resistant is the development of a mutation in the estrogen receptor gene ESR1. ESR1-mutated tumors have constitutive activation of the estrogen receptor and patients who develop these mutations have a worse prognosis; these patients need novel treatment options.
Selective estrogen receptor degraders (SERDs) treat estrogen receptor–positive breast cancer by binding to the receptor and causing its degradation and subsequent downregulation. Currently, intramuscularly delivered fulvestrant is the only SERD approved by the U.S. Food and Drug Administration, but a number of oral SERDs, such as camizestrant, are in development.
“Next-generation oral SERDs have the potential to shut down the growth signaling derived from a constitutively activated estrogen receptor, which is especially important in the setting of tumors with ESR1 mutations,” she said. In this study, treatment with camizestrant at both doses reduced levels of mutated ESR1 to undetectable or near-undetectable levels much more effectively than fulvestrant.
About SERENA-2
In the phase II SERENA-2 trial, 240 previously treated patients with advanced hormone receptor–positive, HER2-negative advanced breast cancer deemed candidates for fulvestrant monotherapy were randomly assigned to receive oral camizestrant at 75 mg or 150 mg daily or fulvestrant at 500 mg by intramuscular injection every 4 weeks. (An arm evaluating camizestrant at 300 mg daily was discontinued after only 20 patients were enrolled.)
Approximately one-third of patients had ESR1 mutations, and almost 60% had visceral metastases. All patients had had disease progression after at least one endocrine therapy in the advanced setting, and approximately half the patients also had received a CDK4/6 inhibitor.
Camizestrant Improves Progression-Free Survival
Median progression-free survival by investigator assessment in patients who received 75 mg or 150 mg of camizestrant doubled that achieved with fulvestrant (Table 1). “In the overall population, camizestrant produced a statistically significant and clinically meaningful improvement in progression-free survival for both doses over fulvestrant. Blinded independent central assessment, conducted as a sensitivity analysis, also showed a statistically significant benefit at both doses, over fulvestrant, consistent with the investigator assessment,” Dr. Oliveira reported.
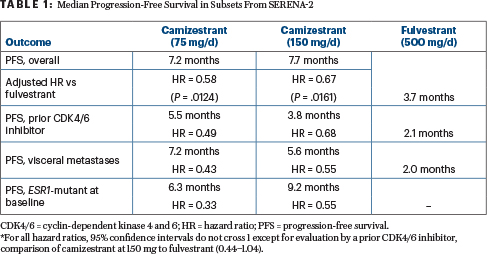
Camizestrant was heavily favored in subsets of patients with visceral metastases, those with detectable ESR1 mutations, and those with evidence of estrogen receptor–driven disease (Table 1). In patients with an ESR1 mutation, believed to confer resistance to endocrine therapy, the median progression-free survival was 6.3 months with 75 mg of camizestrant, 9.2 months with 150 mg, and 2.2 months with fulvestrant.
Changes were also observed in ESR1 mutation variant allele frequency in circulating tumor DNA. “Treatment with camizestrant of both doses reduced the level of ESR1 mutation to undetectable or near-undetectable levels by cycle 2 day 1 and maintained these lower levels for at least 6 months. While fulvestrant also reduced these levels, it was not to the same extent as camizestrant,” she said.
Response rates were numerically higher with camizestrant: 15.7% with 75 mg and 20.3% with 150 mg, compared with 11.5% with fulvestrant. The clinical benefit rates at 24 weeks were 48.8%, 51.0%, and 39.1%, respectively.
Tolerability of Camizestrant
“Both camizestrant doses are well tolerated, with infrequent grade 3 or higher treatment-related adverse events, dose reductions, and discontinuations,” said Dr. Oliveira. More adverse events, however, did occur with camizestrant than with fulvestrant, including more grade ≥ 3 adverse events. However, as she pointed out, “the proportion of grade 3 or higher events with camizestrant is extremely low,” at 12.2% of those receiving 75 mg/d, 21.9% of those given 150 mg/d, and 13.7% of those treated with fulvestrant.
An unusual ocular side effect, photopsia (visual distortions such as floaters and flashes of light), was seen (all grades) in 12.2% of patients receiving 75 mg/d of camizestrant and 24.7% of those receiving 150 mg/d. However, photopsia was not reported in fulvestrant-treated patients.
“We started to see this in the phase I trial, and it seems dependent on dose. But it is extremely important to note that these events are grade 1—basically, flashes of light that do not interfere with vision or patients’ daily activities,” Dr. Oliveira explained. “The mechanism is not yet clear.”
Other Clinical Trials
Enrollment is continuing for two phase III studies of camizestrant in advanced breast cancer—SERENA-4 and SERENA-6— both of which evaluate a dose of 75 mg/d in combination with a CDK4/6 inhibitor.
Camizestrant is one of multiple oral SERDs in clinical development. The agent furthest along in development is elacestrant, which might soon gain U.S. Food and Drug Administration approval based on an update presented in San Antonio.2 Another interesting compound is ARV-471, a selective, orally administered proteolysis targeting chimera (PROTAC) protein degrader, also described in San Antonio.3 ARV-471 targets both wild-type and mutant estrogen receptors by directly binding to an E3 ubiquitin ligase, resulting in proteasomal degradation of the estrogen receptors. In 71 patients in the phase II expansion cohort of the -VERITAC study, the clinical benefit rate at 24 weeks was 38%.
DISCLOSURE: The SERENA-2 study was funded by AstraZeneca. Dr. Oliveira has served as a consultant or on the speakers bureau for AstraZeneca, Gilead Sciences, GSK, iTEOS, MSD, Pierre Fabre, Roche, SeaGen, Eisai, Novartis, and Roche.
REFERENCES
1. Oliveira M, Pominchuk D, Nowecki Z, et al: Camizestrant, a next-generation oral SERD, vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: Results of the randomized, multi-dose phase 2 SERENA-2 trial. 2022 San Antonio Breast Cancer Symposium. Abstract GS3-02. Presented December 8, 2022.
2. Bardia A, Bidard FC, Neven P, et al: EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2– metastatic breast cancer: Updated results by duration of prior CDK4/6i in metastatic setting. 2022 San Antonio Breast Cancer Symposium. Abstract GS3-01. Presented December 8, 2022.
3. Hurvitz SA, Ma C, Hamilton E, et al: ARV-471, a PROTAC estrogen receptor degrader in advanced ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer: Phase 2 expansion (VERITAC) of a phase 1/2 study. 2022 San Antonio Breast Cancer Symposium. Abstract GS3-03. Presented December 8, 2022.

