In a phase II study of stage IV non–small cell lung cancer (NSCLC), domvanalimab, a novel antibody that blocks T-cell immunoglobulin and ITIM domain (TIGIT), when added to anti–PD-1 zimberelimab immunotherapy resulted in improved response rates and progression-free survival compared with zimberelimab alone. These findings were presented during the December ASCO Plenary Series by Melissa L. Johnson, MD, Director of Lung Cancer Research, Sarah Cannon Research Institute, Tennessee Oncology, Nashville.1

Melissa L. Johnson, MD

Lauren Byers, MD
“Specifically, TIGIT combinations resulted in approximately a 13% [absolute] improvement in objective response rate and approximately a 40% reduction in risk of disease progression or death, with a substantial improvement in median progression-free survival,” Dr. Johnson said. “We are particularly encouraged by the number of patients benefiting at 6 months, as evidenced by tumors being stable or shrinking. It should be noted that this is an interim analysis, and the data will continue to mature with longer follow-up.”
Thoracic oncologist Lauren Byers, MD, commented on these findings: “There have been mixed signals from prior studies looking at TIGIT targeting. The promising results of the combination immunotherapy in this study support further investigation. If they are confirmed, it could lead to a new standard of care for patients with advanced lung cancer.”
Why This Regimen?
The phase II ARC-7 trial evaluated the addition of two novel agents—domvanalimab and etrumadenant—to the immunotherapeutic agent zimberelimab in advanced NSCLC. Domvanalimab is an Fc-silent humanized IgG1 monoclonal antibody that blocks TIGIT, thereby reducing immunosuppression of T cells and natural killer cells and promoting antitumor activity. Zimberelimab is a monoclonal antibody directed against PD-1. Etrumadenant is a selective dual A2aR and A2bR adenosine receptor antagonist expressed on immune cells, thereby reducing immunosuppressive extracellular adenosine and potentially modulating immune response.
Of note, TIGIT and PD-1, which is targeted by zimberelimab, have distinct functions in controlling antitumor immune responses, suggesting their combination could be beneficial. Previous TIGIT-directed agents have shown mixed benefit in clinical trials, but domvanalimab, unlike other TIGIT-targeted drugs, is designed to avoid depleting peripheral immune cells, as they can be key to a robust immune response, Dr. Johnson explained. Dr. Johnson and her colleagues evaluated whether the inhibition of TIGIT and adenosine pathways may augment the activity of zimberelimab as a first-line treatment of PD-L1–high, stage IV NSCLC.
About ARC-7
ARC-7 enrolled 150 patients with previously untreated, stage IV NSCLC who had high expression of PD-L1 (tumor proportion score [TPS] ≥ 50%) and no EGFR or ALK alterations. They were randomly assigned to one of three treatment regimens:
- Arm Z: Zimberelimab at 360 mg intravenously every 3 weeks. Patients with disease that progressed were allowed to cross over to arm EDZ.
- Arm DZ: Domvanalimab at 15 mg/kg intravenously every 3 weeks plus zimberelimab.
- Arm EDZ: Etrumadenant at 150 mg orally once a day, in addition to the DZ regimen.
The study’s co-primary endpoints were overall response rate and progression-free survival. Efficacy for this interim analysis was based on 133 patients randomly assigned at least 13 weeks prior to data cutoff, allowing for at least two postbaseline scans.
Improved Outcomes in Domvanalimab Arms
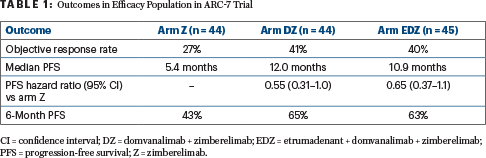
After a median follow-up of 11.8 months, both arms containing domvanalimab—DZ and EDZ—demonstrated improved response rates and progression-free survival as compared with zimberelimab alone (Table 1), Dr. Johnson reported. Objective response rates were 27% in arm Z, 41% in arm DZ, and 40% in arm EDZ. Median progression-free survival was 5.4 months in arm Z, 12.0 months in arm DZ, and 10.9 months in arm EDZ. At 6 months, progression-free survival rates were 43%, 65%, and 63%, respectively.
At the time of data cutoff, 12 patients received crossover treatment with EDZ. A total of 17% of these patients responded, and 67% achieved stable disease; five remained on this treatment, reported Dr. Johnson.
Grade ≥ 3 treatment-related adverse events occurred in about half of all groups, specifically in 58% of arm Z, 47% of arm DZ, and 52% of arm EDZ. All cases of rash were grade 1 or 2, were manageable with topical corticosteroids, and were more common with EDZ. The addition of domvanalimab to zimberelimab did not significantly increase the occurrence of immune-related adverse events, which were seen in 47% receiving the two drugs and 48% receiving zimberelimab alone. Low rates of infusion-related reactions were observed (4%–10%), “as intended with the Fc-silent design of domvanalimab,” she said.
With proof-of-concept established by this phase II trial, several global phase III trials are now in progress to confirm the activity of domvanalimab combination treatments in different types of NSCLC: ARC-10, STAR-121, and PACIFIC-8.
DISCLOSURE: Dr. Johnson has served as a consultant or advisor to (all institutional) Genentech/Roche, Boehringer Ingelheim, AstraZeneca, Calithera Biosciences, Merck, Sanofi, Mirati Therapeutics, Ribon Therapeutics, AbbVie, GlaxoSmithKline, Gritstone Bio, Janssen Oncology, Lilly, Amgen, Bristol Myers Squibb, Daiichi Sankyo, EMD Serono, G1 Therapeutics, WindMIL, Axelia Oncology, Black Diamond Therapeutics, CytomX Therapeutics, EcoR1 Capital, Editas Medicine, Genmab, Ideaya Biosciences, ITeos Therapeutics, Oncorus, Regeneron, Turning Point Therapeutics, and Astellas Pharma. Dr. Byers has received honoraria from or served as a consultant or advisor to UpToDate, Clinical Care Options, Merck Sharp & Dohme, Arrowhead Pharmaceuticals, Chugai Pharmaceutical, AstraZeneca, Genentech, AbbVie, BeiGene, and Jazz Pharmaceuticals.
REFERENCE
1. Johnson ML, Fox W, Lee YG, et al: ARC-7: Randomized phase 2 study of domvanalimab + zimberelimab ± etrumadenant vs zimberelimab in first-line, metastatic, PD-L1–high non–small cell lung cancer. ASCO Plenary Series. Abstract 397600. Presented December 20, 2022.


