The primary outcome analysis of the phase III monarchE trial, an update of previous data, continued to show significant benefit for abemaciclib in the adjuvant setting, reducing the risk for invasive disease recurrence by 28.7%. Meanwhile, the first results of the phase III PENELOPE-B trial of palbociclib in early breast cancer found no benefit. These divergent results were presented at the 2020 San Antonio Breast Cancer Symposium.1,2
PENOLOPE-B builds upon similar results shown for adjuvant palbociclib in the previous PALLAS study.3 At a median follow-up of 24 months, invasive disease–free survival was approximately 88% in both arms, and distant relapse–free survival was around 90%.
Breast cancer specialists are left scratching their heads. Why are outcomes different for two drugs of the same class that show equal benefit in the metastatic setting? Both drugs are inhibitors of cyclin-dependent kinases 4 and 6 (CDK4/6) and are approved for advanced breast cancer. As yet, there is no real answer.
Update on monarchE
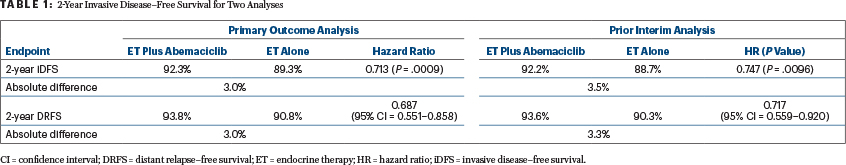
The primary outcome analysis1 was conducted after an extended follow-up of the monarchE trial, for which an interim analysis presented at the European Society for Medical Oncology (ESMO) Congress 2020 showed a significant benefit.3 In both analyses, abemaciclib as an adjunct to endocrine therapy significantly reduced the risk for invasive disease recurrence in women with early-stage hormone receptor–positive, HER2-negative breast cancer (Table 1).
The study was reported at the San Antonio meeting by Priya Rastogi, MD, Associate Professor of Medicine at the University of Pittsburgh School of Medicine/UPMC Hillman Cancer Center, and Medical Director of NSABP Foundation, who noted: “Abemaciclib in combination with endocrine therapy is the first CDK4/6 inhibitor to demonstrate efficacy and tolerability in the early breast cancer population.” The first author was Joyce A. O’Shaughnessy, MD, the Celebrating Women Chair in Breast Cancer Research, Baylor University Medical Center, and Chair of the Breast Cancer Program, Texas Oncology, and the US Oncology Network, Dallas.
The data reflect an additional 3.6 months of follow-up, 72 additional events among the population of 5,637 patients, and an analysis of outcomes in the 2,498 patients with Ki67-high tumors. It was performed after 395 events and a median follow-up of 19 months.
In the new analysis, abemaciclib plus standard endocrine therapy significantly reduced the risk for invasive disease recurrence or death by 28.7%, compared with endocrine therapy alone (hazard ratio [HR] = 0.713; 2-sided P = .0009). Invasive disease–free survival rates at 2 years were 92.3% and 89.3%, respectively. Improvements in distant relapse–free survival were also observed, as was a significant benefit in patients with Ki67-high tumors (Table 1).
In the Ki67-high population, the invasive disease–free survival risk at 2 years was reduced by 30.9% with abemaciclib (HR = 0.691; 2-sided P = .0111). The respective invasive disease–free survival rates were 91.6% and 87.1%—a 4.5% absolute difference.

Priya Rastogi, MD

Joyce A. O’Shaughnessy, MD
More About monarchE
In the monarchE trial, patients with hormone receptor–positive/HER2-negative, node-positive, high-risk early breast cancer were enrolled based on clinicopathologic risk factors (four or more positive axillary lymph nodes or one to three nodes and grade 3 histology or a tumor size ≥ 5 cm) or Ki67 status (one to three to positive nodes, Ki67 index ≥ 20%, no grade 3 histology, and tumor size < 5 cm). The intent-to-treat population of 5,637 subjects included patients in both cohorts.
They were randomly assigned to standard endocrine therapy for 5 to 10 years as clinically indicated, alone or with abemaciclib at 150 mg twice daily for up to 2 years. The primary endpoint of the trial was invasive disease–free survival.
The safety data on the abemaciclib regimen are consistent with the findings reported in the second interim analysis. The most common toxicities were diarrhea, fatigue, and neutropenia. Rare cases of interstitial lung disease and venous thromboembolism were observed.
Findings From PENELOPE-B
The phase III PENELOPE-B trial evaluated the benefit of treating patients deemed to be at high risk for recurrence after neoadjuvant therapy with 1 year of palbobiclib added to endocrine therapy. The approach failed to improve invasive disease–free survival over endocrine therapy alone, according to Sibylle Loibl, MD, PhD, Chair of the German Breast Group.2

Sibylle Loibl, MD, PhD
“This is the first study showing the mature invasive disease–free survival results on a CDK4/6 inhibitor as part of adjuvant therapy [in patients who received neoadjuvant therapy]. To date, the results of PENELOPE-B do not support the addition of 1 year of palbociclib to endocrine therapy,” Dr. Loibl said. “It could be the treatment duration of 1 year is too short, but we don’t know that.”
Starting in 2014, the phase III trial enrolled 1,250 women (median age, 49) with hormone receptor–positive, HER2-negative primary breast cancer considered to be at high risk for recurrence after neoadjuvant therapy. This was determined by a staging system based on pretreatment clinical stage, final pathologic stage, estrogen receptor status, and nuclear grade. CPS-EG (clinical-pathologic scoring system plus estrogen receptor status and grade) staging is considered to be more robust prognostically than pathologic stage alone. Patients in the study had a CPS-EG score ≥ 3 (59%) or ≥ 2 with positive lymph nodes (41%).
Patients received neoadjuvant chemotherapy and surgery, plus or minus radiotherapy. Then they were randomly assigned to 1 year of endocrine therapy plus palbociclib at 125 mg daily or placebo.
At a median follow-up of 43 months, the 3-year invasive disease–free survival rate was 81.2% with palbociclib and 77.7% with placebo (HR = 0.93; P = .525, 2-sided); at 4 years, it was 73.0% and 72.4%, respectively. These events were distant recurrences in 74% and invasive locoregional recurrences in 16%. No subgroup derived benefit, including patients with high Ki67 levels.
“We would have seen a large difference at 2 years (4.3%), but the trial was not stopped for futility,” Dr. Loibl said. “We were disappointed to see the curves come together and even cross around 4 years.”
No overall survival difference was observed, with survival rates of 90.4% with palbociclib and 87.3% with placebo (HR = .087; P = .420).
Adverse events grade ≥ 3 were observed in 80% of the palbociclib arm vs 20% of the placebo arm (P < .001), but serious adverse events were similar (around 9%). In total, 80.5% of patients receiving palbociclib completed treatment, compared with 84.5% of patients receiving placebo. “Although compliance was lower in the palbociclib arm, it was still satisfactory, and the relative total dose intensity was over 80%,” Dr. Loibl said.
“Long-term follow-up from all adjuvant CDK4/6 inhibitor studies should continue and must be awaited,” Dr. Loibl commented.
DISCLOSURE: Dr. Rastogi has served on the advisory board (uncompensated) of Genentech/Roche and has received travel support from AstraZeneca, Genentech/Roche, and Eli Lilly. Dr. O’Shaughnessy has served as a consultant for AbbVie, Agendia, Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Eisai, Genentech, Genomic Health, GRAIL, Immunomedics, Heron, Ipsen, Jounce, Lilly, Merck, Myriad, Novartis, Ondonate, Pfizer, Puma, Prime Oncology, Roche, Seattle Genetics, Syndax Pharma, and Takeda. Dr. Loibl has received honoraria from Prime and Chugai as well as numerous institutional research grants.
REFERENCES
1. O’Shaughnessy JA, Johnston S, Harbeck N, et al: Primary outcome analysis of invasive disease-free survival for monarchE. 2020 San Antonio Breast Cancer Symposium. Abstract GS1-01. Presented December 9, 2020.
2. Loibl S, Marme F, Martin M, et al: Phase III study of palbociclib combined with endocrine therapy in patients with hormone-receptor-positive, HER2-negative primary breast cancer and high relapse risk after neoadjuvant chemotherapy: First results from PENELOPE-B. 2020 San Antonio Breast Cancer Symposium. Abstract GS1-02. Presented December 9, 2020.
3. Johnston SRD, Harbeck N, Hegg R, et al: LBA5_PR: Abemaciclib combined in high risk early breast cancer. Ann Oncol 31:S1143-S1144, 2020.

