An exploratory analysis of the ExteNET study of neratinib in early HER2-positive breast cancer after treatment with trastuzumab (Herceptin) upheld the findings previously reported for the 2-year analysis, according to the study’s principal investigator Arlene Chan, MD, of the Breast Cancer Research Centre, Western Australia, and Curtin University in Perth.1
“The purpose of presenting results at this meeting is to demonstrate to the oncology community that with ongoing follow-up, we are seeing a consistent benefit for neratinib,” Dr. Chan said at the 2015 San Antonio Breast Cancer Symposium.
“We see the most benefit in three populations: patients with centrally confirmed HER2-positive disease, those with hormone receptor–positive disease, and those who commence neratinib shortly after completing 1 year of adjuvant trastuzumab,” she reported.
Neratinib is an oral tyrosine kinase inhibitor of HER1, HER2, and HER4 that has shown activity in preclinical models, in patients previously treated with trastuzumab, and in those naive to trastuzumab therapy. It is believed that neratinib might help prevent relapse after stopping trastuzumab, which is observed in about one-quarter of patients with HER2-positive early breast cancer.
ExteNET Details
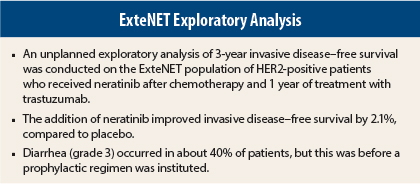
ExteNET enrolled 2,840 HER2-positive patients after chemotherapy and 1 year of trastuzumab treatment. Patients were node-positive or had residual disease after neoadjuvant therapy and thus were considered at high risk for relapse. Half received 12 months of neratinib (140 mg/d) and half, placebo.
In the primary analysis of ExteNET, HER2-positive breast cancer patients who received neratinib for 1 year following adjuvant chemotherapy plus trastuzumab had a 2.3% absolute improvement in invasive disease–free survival at 2 years (P = .009), she reported at the 2015 ASCO Annual Meeting.2
The differences were statistically significant in the hormone receptor–positive subset (hazard ratio [HR] = 0.51, P = .001), though the hormone receptor–negative subset saw no benefit.
Rationale for Exploratory Analysis
“Due to the change in the trial’s sponsor in 2011, the study stopped recruitment after 2,840 patients, and follow-up was truncated at 2 years. Subsequently, the current sponsor reopened the design of the study, thus allowing us to “re-consent” patients and gather further information that is the basis of the current presentation and subsequent analyses,” Dr. Chan explained.
The current unplanned, exploratory 3-year analysis of invasive disease–free survival included 1,778 patients, which accounts for 85% of the patients at risk. The arms were well balanced except for slightly more node-negative patients in the placebo arm.
The hazard ratios are based on 3-year data, the P values are unadjusted for multiplicity and shown for descriptive purposes only, and Kaplan-Meier curves are drawn to 4 years, she said.
Patients were recruited based on local HER2 testing. Central HER2 testing was performed on 2,041 patients, of whom 84% were confirmed to be HER2-positive and 16% were HER2-negative.
Consistent Benefit at 3 Years
“In the 3-year invasive disease–free analysis, we see again a consistent benefit in those patients receiving neratinib, extended up to 4 years,” Dr. Chan reported. “We thought this analysis was important to show. It demonstrates a consistent benefit from neratinib beyond the 2-year analysis.”
At 3 years, invasive disease–free survival rates were 90.5% in the neratinib arm and 88.6% in the placebo arm (HR = 0.74, P = .023).
In the predefined subgroups, again, significant benefit after longer follow-up was observed among patients whose trastuzumab therapy was completed within 1 year from study entry (HR = 0.72, P = .020), those with centrally confirmed HER2-positive disease (HR = 0.70, P = .037), and those with hormone receptor–positive disease (HR = 0.57, P = .003). Patients with hormone receptor–negative disease did not benefit from neratinib.
Substantial benefit was observed among the patients who both enrolled within 1 year of trastuzumab therapy and who were hormone receptor–positive; their invasive disease–free survival at 3 years was 92.0%, vs 86.9% without neratinib (HR = 0.57, P = .004). Similarly, patients who were hormone receptor–positive and centrally confirmed to be HER2-positive had a 3-year invasive disease–free survival of 94.3%, vs 86.8% without the drug (HR = 0.40, P < .001).
“This illustrates the clinical scenarios where delayed adjuvant neratinib would be of benefit,” Dr. Chan commented.
Dealing With Diarrhea
The main adverse event was diarrhea, which was grade 3 in 39.9% of patients. Median duration of diarrhea was 5 days, and most occurred within the first month of treatment. Quality-of-life worsening (as measured by FACT‑B questionnaire) was observed at month 1, but these differences abated thereafter, she noted.
At the time of the study design, there was no protocol-mandated antidiarrheal prophylaxis in place, but it is now known that an intensive loperamide prophylactic regimen can reduce the rates of grade 3 diarrhea to 17% or lower. Better patient education may lower this rate even more, she suggested.
A prospective phase II trial is evaluating the efficacy of diarrhea prophylaxis in patients receiving neoadjuvant neratinib. ■
Disclosure: Dr. Chan has had a consulting or advisory role with Pfizer, Amgen, and Eisai.
References
1. Chan A, Delaloge S, Holmes FA, et al: Neratinib after trastuzumab-based adjuvant therapy in early-stage HER2+ breast cancer: 3-year analysis from a phase 3 randomized, placebo-controlled, double-blind trial (ExteNET). 2015 San Antonio Breast Cancer Symposium.
Abstract S5-02. Presented December 11, 2015.
2. Chan A, Delaloge S, Holmes F, et al: Neratinib after adjuvant chemotherapy and trastuzumab in HER2-positive early breast cancer: Primary analysis at 2 years of a phase 3, randomized, placebo-controlled trial (ExteNET). 2015 ASCO Annual Meeting. Abstract 508. Presented June 1, 2015.