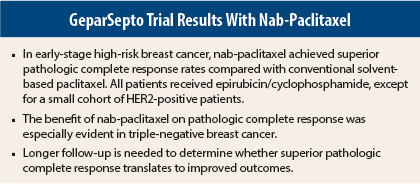
Nab-paclitaxel (Abraxane) achieved superior results compared with conventional solvent-based paclitaxel in patients with early-stage high-risk breast cancer in the large phase III GeparSepto trial from the German Breast Group (GBG).1
The study, presented at the 2014 San Antonio Breast Cancer Symposium, showed a clinically meaningful and statistically significant absolute 9% improvement in pathologic complete response with neoadjuvant nab-paclitaxel compared with conventional paclitaxel, both followed by epirubicin/cyclophosphamide.
With strict criteria, pathologic complete response was 38% with nab-paclitaxel vs 29% with conventional paclitaxel (P < .01). Since pathologic complete response after neoadjuvant therapy is a surrogate marker for long-term efficacy, these results suggest that using nab-paclitaxel instead of conventional paclitaxel may improve outcomes.
“This head-to-head comparison of weekly nab-paclitaxel vs conventional paclitaxel followed by epirubicin/cyclophosphamide showed superiority of nab-paclitaxel in achieving pathologic complete response in early-stage high-risk breast cancer,” said lead author Michael Untch, MD, Chief Physician at Helios Hospital Berlin-Buch, Berlin. “Long-term follow-up will be needed to determine the effect on outcomes.”
Triple-Negative Disease
Subgroup analysis of pathologic complete response showed a consistent and significant benefit in favor of nab-paclitaxel, irrespective of biologic subtypes. The effect of nab-paclitaxel was especially robust in triple-negative breast cancer in this trial, with an almost doubling of pathologic complete response in this specific high-risk subtype (48% vs 25%).
Triple-negative disease was a major driver,” he said. “The odds ratio for pathologic complete response in favor of nab-paclitaxel was 1.53, which was even better than expected. In triple-negative breast cancer, the odds ratio was 2.69,” Dr. Untch told listeners.
The finding in triple-negative breast cancer is especially important, as pathologic complete response is thought to be the most prognostic for outcome in this subtype, which comprises about 15% of all breast cancers.
Study Details
Nab-paclitaxel is engineered to encapsulate paclitaxel in near nanoseized albumin protein shells. This formulation enables delivery of higher dose intensity to the tumor bed compared with conventional paclitaxel, with similar tolerability. Studies have shown that this drug is superior to standard paclitaxel in the metastatic setting.2,3
More than 1,200 patients were treated with four cycles of chemotherapy on two different schedules: nab-paclitaxel for 12 weeks or conventional paclitaxel. In a window-of-opportunity study, patients with HER2-positive tumors (n = 71) received 6 weeks of pertuzumab (Perjeta) vs trastuzumab (Herceptin) or both drugs instead of conventional chemotherapy.
To enter the trial, patients could have unilateral or bilateral or operable or inoperable breast cancer. Tissue samples were obtained for central testing of HER2, hormone receptor status, Ki67, and SPARC testing.
Demographic and disease characteristics were well balanced for the two arms of the trial. The median age was 50 years, the median tumor size was 3 cm, two-thirds of patients were HER2-negative, and about 20% of patients were Ki67-positive.
A Glimpse at Toxicity
Therapy as planned was possible in 79% of the nab-paclitaxel group and 86% of the paclitaxel group. No major differences in hematologic toxicities were observed between the two arms, with the exception of more neutropenia in the nab-paclitaxel arm. Febrile neutropenia was reported in about 4% of both arms.
Sensory peripheral neuropathy was also increased in the nab-paclitaxel arm. Since this was a late-breaking abstract, the recovery from peripheral neuropathy had not yet been analyzed. “The literature suggests that peripheral neuropathy resolves more quickly after nab-paclitaxel compared to paclitaxel,” Dr. Untch noted.
“This study met the primary endpoint of increased pathologic complete response, and the effect of nab-paclitaxel was seen across all subgroups, especially triple-negative breast cancer. Longer term follow-up is needed to see whether increased pathologic complete response translates to improved overall survival,” he said. “It is possible that lower doses could reduce peripheral neuropathy.” ■
Disclosure: This study was sponsored by Roche and Celgene. Dr. Untch reported no potential conflicts of interest.
References
1. Untch M, et al: 2014 San Antonio Breast Cancer Symposium. Abstract S2-07. Presented December 10, 2014.
2. Gradishar WJ, et al: J Clin Oncol 27:3611-3619, 2009.
3. Gradishar WJ, et al: J Clin Oncol 23:7794-7803, 2005.