A randomized trial that sought to determine the optimal approach to treating locally advanced esophageal or gastroesophageal junction cancer has ended in “equipoise,” according to the investigators of the Neo-AEGIS trial. In a study reported at the 2023 ASCO GI Cancers Symposium, perioperative chemotherapy (without radiotherapy) and a radiotherapy-containing multimodal therapy resulted in similar overall survival.1
Patients assigned to chemotherapy before and after surgery, ie, the study’s Neo-AEGIS perioperative regimen, had a 3-year overall survival of 55%, compared with 57% for patients who received the multimodal CROSS regimen of chemotherapy, radiation therapy, and surgery. Despite similar overall survival, rates of pathologic complete response, major pathologic response, clear surgical margins, and nodal downstaging all favored multimodal therapy (Table 1), reported Maeve Lowery, MD, of Trinity College Dublin in Ireland. She suggested that pattern-of-recurrence data could be informative as to why these “proxies of oncologic outcome” did not translate into a survival advantage with multimodal therapy.
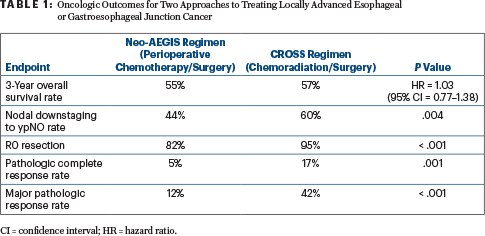
“There was no winner…. This randomized controlled clinical trial reveals no evidence that perioperative chemotherapy is unacceptably inferior to multimodal therapy in the primary outcome of overall survival, notwithstanding greater proxy markers of local tumor response in the CROSS [multimodal therapy] arm. Oncologic and operative outcomes were consistent with optimal modern benchmarks. These data strongly suggest noninferiority and support equipoise in clinical decision-making in modern practice,” Dr. Lowery said.
‘Mind-Boggling’ Results
Upon hearing the presentation, David H. Ilson, MD, PhD, of Memorial Sloan Kettering Cancer Center, New York, shared these comments: “We clearly see equipoise between the two approaches, but what is striking to me is that just five doses of carboplatin and paclitaxel as chemotherapy [radiotherapy was also given] achieved the same survival as around 4 months of chemotherapy. So, what does that tell us about what chemotherapy accomplishes and what should be the new directions, looking at other systemic therapies? To me, it’s really mind-boggling,” he said.

David H. Ilson, MD, PhD

Maeve Lowery, MD
“Obviously, we look forward to seeing the recurrence patterns, but the improvement in pathologic outcomes did not seem to translate into a survival benefit…. It really questions whether more chemotherapy is any better,” Dr. Ilson said.
Responding to Dr. Ilson’s comments, Dr. Lowery said that 40% of patients randomly assigned to the Neo-AEGIS regimen received the prescribed adjuvant chemotherapy, and fewer still received all planned doses of adjuvant therapy. “So, I would agree with you completely [about more chemotherapy]…. And we haven’t published quality-of-life data yet, but it’s clear that patients were happy to be done [with treatment] after a short period,” she said.
Dr. Ilson also congratulated the Neo-AEGIS investigators on performing an “important and pivotal” study. “I think this also stresses the importance of the quality of the surgery that was done in the trial.”
Study Rationale and Methods
The optimal curative approach to locally advanced adenocarcinoma of the esophagus and gastroesophageal junction remains controversial, specifically whether multimodal therapy or perioperative chemotherapy is superior. In 2012, the CROSS study found that preoperative chemoradiotherapy improved overall survival over surgery alone (hazard ratio [HR] = 0.657; P = .003).2 “The major question then became whether radiation therapy is required where en bloc resection and radical lymphadenectomy is the standard,” Dr. Lowery said.
Neo-AEGIS was the first randomized clinical trial to directly compare these approaches, addressing the question of whether radiotherapy must be given with chemotherapy before complete resection (primarily en bloc transthoracic esophagectomy) and radical lymphadenectomy for esophageal or gastroesophageal junction cancer. The primary endpoint was overall survival, and the trial had statistical power to demonstrate noninferiority.
Investigators randomly assigned 377 patients with stage 2 or 3 disease, tumors up to 8 cm, and any nodal involvement to either of the following regimens:
The Neo-AEGIS regimen of three or four cycles of chemotherapy before and after surgery. From 2013 to 2018, chemotherapy was the modified MAGIC regimen, and from 2019 to 2020, patients received MAGIC or FLOT (15% received FLOT). The modified MAGIC regimen included epirubicin, cisplatin or oxaliplatin, and fluorouracil or capecitabine. The FLOT regimen included fluorouracil, leucovorin, oxaliplatin, and docetaxel.
The CROSS regimen of neoadjuvant chemotherapy and radiotherapy prior to surgery. Treatment involved chemotherapy with carboplatin or paclitaxel plus 41.4 Gy of radiation therapy.
KEY POINTS
- The Neo-AEGIS trial compared two approaches to locally advanced esophageal or gastroesophageal junction cancer, finding one not superior to the other in terms of overall survival.
- Perioperative chemotherapy (without radiation) and multimodal chemoradiation (CROSS regimen) both achieved overall survival rates of around 56% at 3 years.
- Proxy outcomes, such as pathologic complete response and R0 resections, were more likely in the multimodal therapy arm.
The data safety and monitoring committee recommended closure of the study after the second futility analysis in December 2020.
No Difference in Overall Survival
After a median follow-up of 34.2 months, in the primary analysis, no differences were observed in overall survival for the comparison between the Neo-AEGIS and CROSS treatment groups (Table 1). Survival curves overlapped throughout follow-up.
The two groups also did not differ with respect to the frequency or severity of operative complications or postoperative mortality. However, toxicity and grade 3 or 4 adverse events occurred more often with the Neo-AEGIS regimen, primarily neutropenia, diarrhea, and vomiting. “Hence, there was no negative effect of radiotherapy,” Dr. Lowery said.
DISCLOSURE: Dr. Lowery has served as a consultant or advisor to AstraZeneca, Roche/Genentech, and Servier and has received research fundings from Astellas Pharma, Basilea, Exelixis, and MSD. Dr. Ilson has served as a consultant or advisor to Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, IQvia, Lilly/ImClone, MacroGenics, Merck, Pieris Pharmaceuticals, Roche/Genentech, and Taiho Pharmaceutical.
REFERENCES
1. Reynolds JV, Preston SR, O’Neill B, et al: Neo-AEGIS (neoadjuvant trial in adenocarcinoma of the esophagus and esophago-gastric junction international study): Final primary outcome analysis. 2023 ASCO GI Cancers Symposium. Abstract 295. Presented January 19, 2023.
2. van Hagen P, Hulshof MCCM, van Lanschot JJB, et al: Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074-2084, 2012.

