The use of circulating tumor DNA (ctDNA) assays in early-stage colorectal cancer is highly prognostic for recurrence and may help identify patients who could benefit from adjuvant chemotherapy, according to findings from the GALAXY trial, presented at the 2022 ASCO Gastrointestinal Cancers Symposium by investigators from Japan.1 Patients who tested positive for ctDNA 4 weeks after surgery had an 11- to 13-times increased risk for recurrence.
The analysis of ctDNA dynamics revealed that 6-month and 12-month disease-free survival rates differed according to ctDNA status and how it changed over time. Outcomes were significantly different between the “positive to positive” group and the “positive to negative” group, with an almost 16-fold increased risk for recurrence for patients with persistent ctDNA despite adjuvant chemotherapy, reported Masahito Kotaka, MD, PhD, of the Gastrointestinal Cancer Center at Sano Hospital in Kobe, Japan.

“Our study shows that stratifying postoperative treatment decisions using the assay may identify patients likely to benefit from adjuvant chemotherapy across all stages.”— Masahito Kotaka, MD, PhD
Tweet this quote
In addition, adjuvant chemotherapy was able to clear ctDNA by week 24 in 68% of patients who tested positive for ctDNA at 4 weeks after surgery; recurrences were significantly fewer in this group compared with their untreated peers. In fact, in patients with ctDNA positivity, adjuvant chemotherapy yielded better outcomes across all stages.
“Our study shows that stratifying postoperative treatment decisions using the ctDNA assay may identify patients likely to benefit from adjuvant chemotherapy across all stages. We think ctDNA dynamics from 4 weeks to 12 weeks postop could become a new surrogate endpoint beyond disease-free survival,” Dr. Kotaka said.
GALAXY Details
GALAXY was part of the CIRCULATE-Japan platform protocol, which is enrolling patients with clinical stage II to IV resectable colorectal cancer to evaluate the clinical utility of ctDNA analysis for detecting “molecular residual disease” (MRD). Within the study are the GALAXY observational study and two phase III interventional trials: VEGA and ALTAIR.
GALAXY evaluated a personalized tumor-informed ctDNA assay that is based on whole-exome sequencing of tumor tissue and matched normal samples. Blood samples are collected before surgery and at regular intervals after surgery. Investigators can receive the results of the assay in time to enroll patients with residual disease in the interventional trials.
GALAXY enrolled 1,564 patients, of whom 1,040 (the “outcome cohort”) were included in the current analysis. After further exclusions, the group was divided into three cohorts. The first cohort consisted of patients who tested ctDNA-positive following 4 weeks of surgery (n = 183), and in whom ctDNA clearance was analyzed during adjuvant chemotherapy. This group was known as the “clearance cohort.” The second cohort consisted of patients who tested ctDNA-negative (n = 531) 4 weeks after surgery, and the third group was the “dynamics analysis cohort” (n = 838), for which outcomes associated with changes in ctDNA status over the course of treatment were evaluated.
Prognostic Impact of ctDNA Status on Disease-Free Survival
Dr. Kotaka presented data on disease-free survival by ctDNA status at postoperative week 4 in the overall population of patients with pathologic stage I to IV disease and then for patients with pathologic stage II to III disease, after a median follow-up of 11.4 months (Table 1). He reported that patients who tested positive for ctDNA 4 weeks after surgery had an 11- to 13-times increased risk for recurrence.
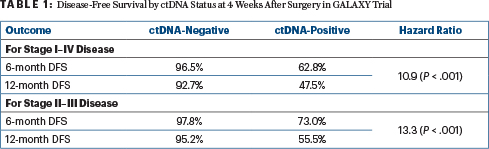
The assay’s sensitivity for disease recurrence was calculated at 63.6% for the analysis of patients with stage I to IV disease and 67.6% for those with stage II to III disease. (Unless otherwise noted, all staging reported in this analysis was pathologically determined.)
The multivariate analysis showed the risk of recurrence for patients with stage II to III tumors was highly correlated with ctDNA positivity at 4 weeks after surgery (hazard ratio [HR] = 15.3; P < .001). Also prognostic for worse outcomes were mutant RAS vs wild-type disease (HR = 1.8; P = .04) and mutant BRAF vs wild-type disease (HR = 5.2; P < .001).
Dynamics Analysis: When ctDNA Status Changes
The dynamics analysis (which excluded patients with recurrence within 12 weeks of surgery) evaluated the predictive effect of ctDNA in this population. Patients with baseline ctDNA positivity who remained positive over the course of treatment had an almost 16-fold increased risk of disease recurrence, Dr. Kotaka reported.
Patients who did not clear their ctDNA between 4 and 12 weeks during adjuvant chemotherapy had significantly worse outcomes relative to those who cleared their ctDNA—ie, “positive to positive” (58.3%) vs “positive to negative” (100%)—exhibiting a 15.8-fold increased risk. The disease-free survival rate for ctDNA-negative patients that remained negative was 98% and for those who turned positive 62.5%.
Impact of Adjuvant Chemotherapy
Taking a closer look at the clearance cohort, wherein -ctDNA-positive patients at 4 weeks postsurgery were administered adjuvant chemotherapy, the impact of treatment assessed as ctDNA clearance was observed to be 68% vs 10% in patients who did not receive adjuvant chemotherapy (HR = 9.3; P < .001). “Interestingly, two of the three patients with ctDNA-positive stage I or low-risk stage II disease experienced recurrence,” he noted. Chemotherapy was mostly with fluoropyrimidine plus oxaliplatin.
In the multivariate analysis of patients with ctDNA-positive stage II to IV disease, risk of recurrence was significantly increased without adjuvant chemotherapy (HR = 5.6; P < .001); patients with RAS-mutant vs wild-type tumors also had worse outcomes (HR = 2.0; P = .03).
For patients with high-risk stage II, stage III, and stage IV disease, adjuvant chemotherapy yielded a benefit among patients who tested positive for ctDNA 4 weeks after surgery, with hazard ratios of 9.4 (P = .04), 8.8 (P < .001), and 2.4 (P = .02), respectively. Patients with high-risk stage II to III disease who tested negative for ctDNA at 4 weeks, on the other hand, had excellent outcomes, whether or not they received chemotherapy, with a disease-free survival of approximately 95% at 12 months.
In the ctDNA-negative cohort, patients with stage III disease were likely to receive adjuvant chemotherapy (83%), whereas this was not the case for patients with high-risk stage II disease (17%), he added.
The ctDNA-guided adjuvant strategy will further be established by the ongoing randomized VEGA and ALTAIR studies and will be presented at future conferences, Dr. Kotaka said.
DISCLOSURE: Dr. Kotaka has received honoraria from Chugai Pharma, Lilly Japan, Taiho Pharmaceutical, Takeda, and Yakult Honsha.
REFERENCE


