As a first-line treatment of intermediate- or high-risk diffuse large B-cell lymphoma, the addition of the antibody-drug conjugate polatuzumab vedotin-piiq to standard-of-care therapy resulted in a 27% reduction in the relative risk of disease progression, relapse, or death, with a similar safety profile.1 The late-breaking results were reported at the 2021 American Society of Hematology (ASH) Annual Meeting & Exposition by Hervé Tilly, MD, of the Centre Henri Becquerel and University of Rouen in France, and published in The New England Journal of Medicine.2 Senior study author Gilles Salles, MD, PhD, Chief of the Lymphoma Service at Memorial Sloan Kettering Cancer Center, presented the findings at a press briefing during the meeting.

Hervé Tilly, MD

Gilles Salles, MD, PhD
“These results support the use of polatuzumab vedotin [plus standard therapy] in the initial management of patients with diffuse large B-cell lymphoma,” Dr. Salles said.
The international double-blind placebo-controlled phase III POLARIX trial evaluated a novel regimen combining the CD79b-targeting antibody-drug conjugate polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) in adults with newly diagnosed diffuse large B-cell lymphoma. For approximately 2 decades, the standard of care has been R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone); however, about 40% of patients are not cured with this strategy.
“Numerous trials have tried to improve upon R-CHOP either by modifying the dose or schedule of a drug within that regimen or by introducing new agents, but, overall, these attempts have been unsuccessful,” Dr. Salles noted.
To address the unmet need for better treatments, POLARIX evaluated the benefit of adding polatuzumab vedotin, which is already approved in combination with bendamustine and rituximab for patients with relapsed or refractory diffuse disease treated with at least two prior therapies.
About POLARIX
POLARIX enrolled a total of 879 patients with previously untreated diffuse large B-cell lymphoma and an International Prognostic Index (IPI) of between 2 and 5. Baseline characteristics were well balanced between the arms. The median patient age was approximately 65 years; almost two-thirds had high-intermediate and high-risk IPI; approximately 40% had double-expressor lymphomas; and around 7% had double- or triple-hit disease.
Investigators randomly assigned 439 patients to the control arm of R-CHOP and 440 to the experimental arm of R-CHP plus polatuzumab vedotin (Pola-R-CHP). Patients received six cycles of these regimens followed by two additional cycles of rituximab.
Doses were polatuzumab vedotin at 1.8 mg/kg, vincristine at 1.4 mg/m2, rituximab at 375 mg/m2, cyclophosphamide at 750 mg/m2, doxorubicin at 50 mg/m2 (on day 1), and oral prednisone at 100 mg once daily (on days 1–5). The primary endpoint was investigator-assessed progression-free survival.
Primary Endpoint Met
After a median follow-up of 28.2 months, progression-free survival was superior with Pola-R-CHP vs R-CHOP (hazard ratio [HR] = 0.73; P < .02). The 24-month progression-free survival rate was 76.7% with the polatuzumab vedotin regimen vs 70.2% with R-CHOP, reflecting an absolute difference of 6.5% (P < .02). Table 1 shows the clinical outcomes at 24 months.
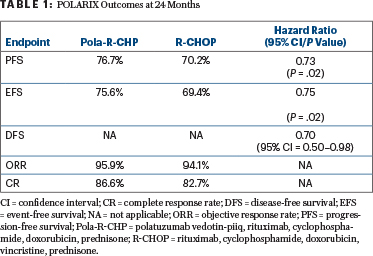
“Because CD79b is ubiquitously expressed on the surface of mature B-cell lymphoid cancers, it is expected that Pola-R-CHP will benefit all patients,” Dr. Salles said in the press briefing. “We observed very significant progress for patients who are more difficult to treat.”
In the publication, the investigators reported that the most benefit was observed in patients older than age 60, in patients who had an IPI score between 3 and 5, and in patients with the activated B-cell–like subtype. The benefit was less clear in patients aged 60 or younger, those with lower IPI scores, patients with bulky disease, and those who had the germinal-center B-cell–like subtype, the study showed.2
No Overall Survival Difference: Effect of Subsequent Therapy?
At the current median follow-up of 28.2 months, no difference in overall survival has emerged, with about 88% of each arm alive at 3 years—a figure that should be “really encouraging for patients,” Dr. Salles said. “We will continue to observe the patients and see whether overall survival curves may diverge over time.”
However, patients with treatment failure on R-CHOP went on to receive many more subsequent therapies than the Pola-R-CHP arm: 30.0% of any kind vs 22.5%. Treatments received after R-CHOP included systemic therapy in 23.5%, radiotherapy in 13.0%, stem cell transplantation in 7.1%, and chimeric antigen receptor (CAR) T-cell therapy in 3.6%.
This “burden of additional treatment” can be costly in many ways, Dr. Salles said. “From the patient’s point of view, going to another line of treatment—and perhaps procedures like transplantation or CAR T-cell therapy—is clearly a burden, despite the potential for good results. If we can avoid that with a first-line treatment, that is significantly beneficial,” Dr. Salles said.
Dr. Tilly, in his presentation of the data at the late-breaking abstracts session, further commented: “The progression-free survival difference is very important, especially in the first-line setting. Overall survival is not different, but progression-free survival is probably the best reflection of the possibility of cure.”
Comparable Safety Profiles
Grade 3 or 4 adverse events were reported in 57.7% of the Pola-R-CHP arm vs 57.5% of the R-CHOP arm. Serious adverse events were seen in 34.0% and 30.6%, and grade 5 events occurred in 3% and 2.3% of patients, respectively. Notably, most cases of peripheral neuropathy were grade 1, and its incidence and severity were similar in the two treatment groups. Rates of neutropenia were also similar. Diarrhea was seen more often with Pola-R-CHP.
Adverse events leading to discontinuation of any study drug occurred in 6.2% of the Pola-R-CHP arm and 6.6% of the R-CHOP arm. Discontinuation of polatuzumab vedotin was recorded for 4.4% and vincristine in 5.0%, respectively, but dose reductions were less frequent in the experimental arm, 9.2% vs 13.0% with standard therapy.
“The safety profiles of polatuzumab vedotin plus R-CHP and R-CHOP were comparable,” Dr. Salles concluded.
DISCLOSURE: Dr. Tilly has served as a consultant or advisor to Karyopharm, F. Hoffmann–LaRoche, AstraZeneca, Janssen-Cilag, and Incyte; has received reimbursement for travel, accommodation, and expenses from F. Hoffmann–LaRoche; and has received research funding from F. Hoffmann–LaRoche. Dr. Salles has received honoraria from Bayer, AbbVie, Allogene, Bristol Myers Squibb/Celgene, Epizyme, Janssen, MorphoSys, and Regeneron; and has served as a consultant or advisor to AbbVie, BeiGene, Bristol Myers Squibb/Celgene, Debiopharm, Epizyme, Roche/Genentech, Genmab, Incyte, Ipsen, Janssen, Kite/Gilead, Loxo, Miltenyi Biotec, MorphoSys, Novartis, Rapt, Regeneron, Takeda, and Velosbio.
REFERENCES
1. Tilly H, Morschhauser F, Sehn L, et al: The POLARIX study: Polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, and prednisone (pola-R-CHP) versus rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) therapy in patients with previously untreated diffuse large B-cell lymphoma. 2021 ASH Annual Meeting & Exposition. Abstract LBA-1. Presented December 14, 2021.
2. Tilly H, Morschhauser F, Sehn LH, et al: Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med 386:351-363, 2022.

