A post hoc analysis of the phase II DREAMM-2 trial showed single-agent belantamab mafodotin-blmf to be efficacious and tolerable in patients with relapsed or refractory multiple myeloma treated with at least three prior therapies, investigators reported at the 2020 American Society of Hematology (ASH) Annual Meeting & Exposition.1
“Belantamab mafodotin achieved deep and durable responses, with no notable alterations in the safety profile, even when patients had received seven or more lines,” said Sagar Lonial, MD, FACP, the Anne and Bernard Gray Family Chair in Cancer, Chair and Professor of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta.

[Belantamab mafodotin] represents a useful treatment option for relapsed or refractory myeloma, including patients with a high burden of prior treatment for whom the prognosis is otherwise poor.— Sagar Lonial, MD, FACP
Tweet this quote
“Efficacy and safety were not impacted by the number of prior therapies. [Belantamab mafodotin], therefore, represents a useful treatment option for relapsed or refractory myeloma, including patients with a high burden of prior treatment for whom the prognosis is otherwise poor,” he said.
About DREAMM-2
The phase II DREAMM-2 trial is an ongoing open-label study examining single-agent belantamab mafodotin, 2.5 or 3.4 mg/kg every 3 weeks, in patients who had received at least three prior lines of treatment and were refractory to an immunomodulating drug or proteasome inhibition.
A previous analysis found deep and durable clinical activity, and a manageable safety profile, with belantamab mafodotin as a single agent.2 The post hoc analysis presented at the ASH meeting looked at the safety and efficacy of belantamab mafodotin at 2.5 mg/kg every 3 weeks according to the number of prior treatments patients received before study entry. The 106 patients were categorized into two subpopulations: those with three to six prior therapies and those with seven or more prior therapies. The primary endpoint was objective response rate.
The safety analysis focused on adverse events of the cornea as determined in regular ocular exams by an eye care professional. To fully characterize ocular events, the study incorporated a
protocol-specified keratopathy visual acuity scale that focused particularly on microcyst-like epithelial changes as observed on slit-lamp exam, with or without symptoms, as well as changes in best corrected visual acuity by the Snellen test.
Patients were well matched in terms of demographics and disease characteristics. All patients in each pretreatment group were refractory to a immunomodulating drug, proteasome inhibitor, and the anti-CD38 antibody daratumumab (ie, were triple-refractory). Many patients had received multiple agents within a given class.
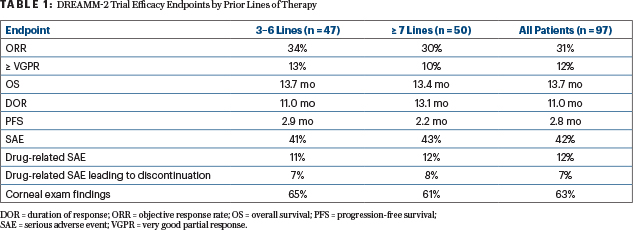
Table 1 displays the efficacy endpoint for the two pretreatment subgroups, showing that overall survival exceeded 13 months in each cohort, and responses were durable. Progression-free survival rates were slightly higher for the less heavily pretreated patients, Dr. Lonial reported (Table 1).
Safety Profile
“The safety profile of [belantamab mafodotin] was similar between the two cohorts, with comparable rates of adverse events, serious adverse events, and dose modifications and comparably low rates of treatment discontinuation,” he reported.
Since ocular events are known to occur with antibody-drug conjugates whose “payload” contains monomethyl auristatin F, they were anticipated and were found to be comparable between the groups in frequency (60%; as determined by the keratopathy visual acuity scale), time to first occurrence, and resolution of last event.
DISCLOSURE: Dr. Lonial has served as a consultant for Juno Therapeutics, Sanofi, Millennium, Genentech, Karyopharm, Novartis, Amgen, Takeda, Merck, AbbVie, GSK, Bristol Myers Squibb, and Janssen; has received honoraria from Onyx, Millennium, Novartis, Amgen, Merck, GSK, Bristol Myers Squibb, and Janssen; has received personal fees from Novartis, Amgen, Takeda, Merck, GSK, Bristol Myers Squibb, and Janssen; has received research funding from Takeda, Bristol Myers Squibb, and Janssen; and has served on a board of directors or advisory committee for TG Therapeutics.
REFERENCES
1. Lonial S, Lee HC, Badros A, et al: DREAMM-2: Single-agent belantamab mafodotin (belamaf) in patients with relapsed/refractory multiple myeloma—1-year outcomes by prior therapies. 2020 ASH Annual Meeting & Exposition. Abstract 1417. Presented December 5, 2020.
2. Lonial S, Lee HC, Badros A, et al: Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207-221, 2020.

