Belantamab mafodotin-blmf combined with pomalidomide and dexamethasone led to a very good partial response (VGPR) or better in approximately three-quarters of patients with multiple myeloma that was double-class or triple-class refractory, according to Suzanne Trudel, MSc, MD, FRCPC, of Princess Margaret Cancer Centre and University of Toronto. She reported the phase I study findings at the 2020 American Society of Hematology (ASH) Annual Meeting & Exposition.1 “The response rate and depth of response were preserved,” Dr. Trudel said, regardless of whether patients were refractory to immunomodulatory agents and proteasome inhibitors and even to daratumumab as well.
The phase I Canada Myeloma Research Group (CMRG) study evaluated belantamab mafodotin, a first-in-class antibody-drug conjugate targeting B-cell maturation antigen (BCMA), with pomalidomide plus dexamethasone (B-Pd). Belantamab mafodotin has a multimodal mechanism of action, including the ability to induce direct cell death by antibody-dependent cellular cytotoxicity, and it retains classical antibody effector functions. The rationale for combining belantamab mafodotin with pomalidomide, an immumodulatory drug, was that the two agents together might enhance antibody-dependent cytotoxicity.

Suzanne Trudel, MSc, MD, FRCPC
“With proteasome inhibitors, immumodulatory drugs, and anti-CD38 antibodies, BCMA-targeted agents are the fourth pillar of myeloma treatment. Belantamab mafodotin is the only anti-BCMA treatment that is readily available for all patients, as it’s off-the-shelf and can be administered in the office without life-threatening toxicities,” Dr. Trudel noted.
Study Details
Dr. Trudel reported the early results of the Algonquin trial, a multicenter Canadian phase I, two-part, dose-escalation study that aims to determine the maximum tolerated dose, recommended phase II dose, safety, tolerability and efficacy of B-Pd in patients who had received at least two prior lines of treatment, were refractory to lenalidomide and exposed to a proteasome inhibitor, and were refractory to their last line of therapy.
Pomalidomide was administered at 4 mg on days 1 to 21, and dexamethasone was given at 40 mg (20 mg age > 75 years) weekly; this regimen was given in conjunction with intravenous belantamab mafodotin in the following dosing schemas: single doses of 1.92 or 2.5 mg/kg given every 4 weeks; doses of 2.5 and 3.4 mg/kg were split equally on days 1 and 8, given every 4 weeks; and a loading dose of 2.5 mg/kg was given for cycle 1, followed immediately by 1.92 mg/kg every 4 weeks from cycle 2 onward.
“The loading dose was based on the assumption that patients with relapsed disease have high levels of soluble BCMA, and we wanted to make sure they received an adequate first dose. We know that soluble BCMA levels dramatically decrease after the first cycle, so we felt comfortable going down to 1.92 mg/kg, which is the minimum required for saturation of BCMA, starting with cycle 2,” Dr. Trudel explained.
At data cutoff, 37 patients were enrolled; the 20 patients receiving 2.5 mg/kg in the single (including loading dose) and split-dosing groups were combined for the analysis. More than 40% had been exposed to daratumumab, 73% were refractory to both lenalidomide and a proteasome inhibitor, and 35% were refractory to those two agents plus daratumumab. Almost half the patients had high-risk cytogenetics. The median number of cycles was nine, and the median follow-up was 7.8 months.
At Least a Very Good Partial Response in Majority
Dr. Trudel reported the response rates and progression-free survival for all 34 evaluable patients, for the dosing subgroups, and by refractoriness (Table 1). In addition, she noted that two of two complete responders became negative for minimal residual disease. A higher depth of response translated into improved progression-free survival.
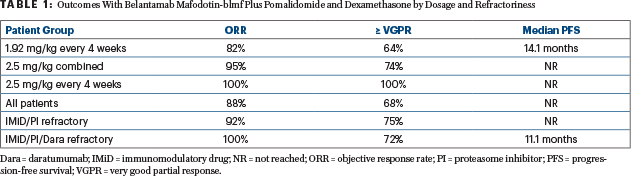
“Comparing clinical activity between the two doses, the response rate and depth of response were higher in patients receiving the initial dose of 2.5 mg plus pomalidomide and dexamethasone, with 74% of these achieving at least a very good partial response and 26% achieving a complete response. If you remove patients who received the 2.5-mg/kg split dose and focus on the 12 who received an initial dose of 2.5 mg/kg and were dosed every 4 weeks, the rate of at least a very good partial response was 100%,” Dr. Trudel reported. “I highlight this because this dosing schedule has been chosen for DREAMM-8, which is comparing B-Pd with pomalidomide/bortezomib and dexamethasone.”
“Although the numbers are small and there are differences in patient characteristics, compared with published trials of pomalidomide/dexamethasone triplets in a population who has received at least two prior lines, B-Pd compares favorably, despite the fact that 43% of our patients had disease refractory to daratumumab,” she commented.
Safety Overview
Three dose-limiting toxicities were observed, all grade 3 keratopathy: one at 2.5 mg/kg and two at 3.4 mg/kg. Thus, the maximum tolerated dose was established as 2.5 mg/kg as a single dose and 2.5 mg/kg as a split dose, in combination with standard dosing of pomalidomide/dexamethasone.
The overall safety profile was consistent with the known toxicities of belantamab mafodotin and pomalidomide. Grade 3 or 4 treatment-related adverse events were observed in 76% of patients; 24% had serious treatment-related adverse events, primarily fever, lung infection, and neutropenia. One patient discontinued treatment because of an adverse event, 38% had dose reductions, and 73% had dose interruptions or delays.
Grade 3 or 4 thrombocytopenia occurred in 32%, and grade 3 or 4 neutropenia was observed in 40%. Infusion-related reactions, primarily grade 1 or 2, were seen in 30%.
Keratopathy Details
KEY POINTS
- A phase I dose-escalation study evaluated the antibody-drug conjugate belantamab mafodotin combined with standard-dose pomalidomide plus dexamethasone.
- Very good partial responses or better were observed in about three-quarters of patients, including those with disease refractory to two and three agents.
- Keratopathy grade ≥ 3 was observed in 51%, but 16% of patients experienced decreased visual acuity grade ≥ 3.
The most common adverse event was keratopathy—a known toxicity of belantamab mafodotin—with 75% of patients developing any grade and 51% experiencing at least grade 3. Keratopathy is the observation on slit lamp exam of corneal microcystic changes associated with blurred vision or decreased visual acuity. Although half the patients had a grade 3 or 4 event, 16% experienced decreased visual acuity of at least grade 3, noted Dr. Trudel.
One patient discontinued treatment because of grade 4 reduction in visual acuity, which recovered to grade 3 within 7 days and eventually resolved to grade 1. Few patients had accompanying pain or photophobia, she added.
Grade 3 keratopathy was more prevalent in the 2.5-mg/kg group than in patients receiving 1.92 mg/kg (70% vs 25%). However, meaningful decreases in best corrected visual acuity (20/50 or worse) were similar, 15% and 17%, respectively. Despite this similar percentage, dose holds were needed more often in the 2.5-mg/kg group (median, five vs one per patient).
“The observation of improved efficacy with 2.5 mg/kg that was preserved despite dose holds has prompted the evaluation of an alternative dosing schedule—2.5 mg/kg given every 8 or 12 weeks—in an effort to optimize efficacy and safety,” Dr. Trudel said.
DISCLOSURE: Dr. Trudel has served as a consultant for or received honoraria from Amgen, Takeda, Sanofi, Karyopharm, AstraZeneca, Pfizer, Janssen, GlaxoSmithKline, and Bristol Myers Squibb.
REFERENCE
1. Trudel S, McCurdy A, Sutherland HJ, et al: Part 1 results of a dose-finding study of belantamab mafodotin (GSK2857916) in combination with pomalidomide and dexamethasone for the treatment of relapsed/refractory multiple myeloma. 2020 ASH Annual Meeting & Exposition. Abstract 725. Presented December 7, 2020.

