To complement The ASCO Post’s continued comprehensive coverage of the 2019 American Society of Hematology (ASH) Annual Meeting & Exposition, here are several abstracts selected from the meeting proceedings focusing on allogeneic and autologous hematopoietic cell transplantation. For full details of these study abstracts, visit ashpublications.org.
GUEST EDITORS

Syed Ali Abutalib, MD

Navneet Majhail, MD, MS
Allogeneic Hematopoietic Cell Transplantation
ABSTRACT 48: Genetic alterations at diagnosis predict outcome of older adults (age ≥ 60) with acute myeloid leukemia (AML) undergoing allogeneic hematopoietic cell transplantation (allo-HCT) in first complete remission: Retrospective multicenter study1
Background: Older age has been associated with inferior outcomes after allo-HCT in AML. An enhanced understanding and analysis of recurrent genetic alterations may provide the basis for improving transplant outcomes in these patients.
Methods: Investigators of the study performed targeted sequencing of 112 genes on diagnostic leukemia samples from 257 patients. About 31% had clinically defined secondary AML, 11% had therapy-related AML, and 23% had adverse cytogenetics according to 2017 ELN (European Leukemia Network) classification. The median ages at diagnosis and at allo-HCT were 65 and 66, respectively. Most patients (84%) were treated with anthracycline-based induction chemotherapy, whereas 16% received nonintensive induction. Conditioning was either at reduced intensity or nonmyeloablative in 94% of patients. The median follow-up for survivors was 3.7 years.
Results: The impact of gene mutations on leukemia-free survival after transplantation using univariable and multivariable Cox models was evaluated.
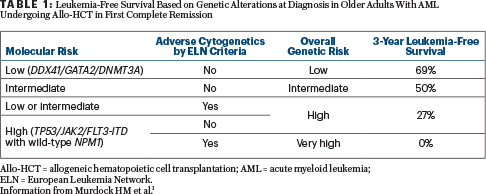
• Hierarchical model of three molecular genetic risk groups: (1) patients with TP53 mutation or JAK2 mutation or FLT3-ITD/NPM1-wild type (high risk); (2) patients without high risk mutations who have DNMT3A or GATA2 or DDX41 mutations (low risk); (3) patients without high- or low-risk mutations (intermediate risk). These groups were associated with 3-year leukemia-free survival rates of 8%, 65%, and 47% (P < .001), respectively.
• Hierarchical model with combined molecular (protocol-defined risk group) and cytogenetic risk (ELN criteria) to derive a final genetic model comprising four groups with distinct 3-year leukemia-free survival (69%, 50%, 27%, and 0%).
Clinical Implications: Genetic characteristics at diagnosis are associated with overall (refer to graphs depicted in the abstract1) and leukemia-free survival in older adults (age ≥ 60) with AML who undergo allo-HCT in first complete remission (Table 1). Validation of these findings will refine risk classification of patients prior to transplantation, including identifying high-risk patients for whom current transplantation modalities may have limited benefit and who are candidates for novel transplant and nontransplant approaches.
ABSTRACT 147: The Path to Cure: Using haploidentical donors and high-dose posttransplant cyclophosphamide for treatment-naive (n = 15) and relapsed or refractory (n = 20) severe aplastic anemia2
Background: Severe aplastic anemia is a life-threatening hematopoietic stem cell disorder that is often treated with allo-HCT using marrow graft to reconstitute hematopoiesis and eliminate clonality. Given that the risk of relapse or secondary clonal disease following immunosuppressive therapy is more than 50% at 5 years, investigators utilized a haploidentical marrow grafts and posttransplant cyclophosphamide paradigm in treatment-naive and relapsed or refractory (at least one course of immunosuppressive therapy) severe aplastic anemia.
Methods: The conditioning regimen included a standard published haploidentical backbone of antithymocyte globulin, low-dose cyclophosphamide, and total-body irradiation. All patients with relapsed or refractory disease received 200 cGy. The dose of total-body irradiation for the treatment-naive cohort was modified after three graft failures in the initial seven patients treated with a total-body irradiation dose of 200 cGy; the next eight treatment-naive patients received 400 cGy. After marrow graft infusion on day 0, cyclophosphamide at 50 mg/kg/d was administered intravenously on days +3 and +4 posttransplant. All patients received mycophenolate mofetil beginning day +5 through +35 and tacrolimus on day +5 to +365.
Results: All 20 patients with relapsed or refractory disease were alive, free of disease, and with no evidence of active graft-vs-host disease (Table 2). The increase in the dose of total-body irradiation to 400 cGy in treatment-naive patients appears to achieve more durable engraftment without increasing the risks of early toxicity (infection, delayed engraftment, graft-vs-host disease; Table 2). More patients and longer follow-up are required to understand the implications of such treatment on fertility and other survivorship issues.
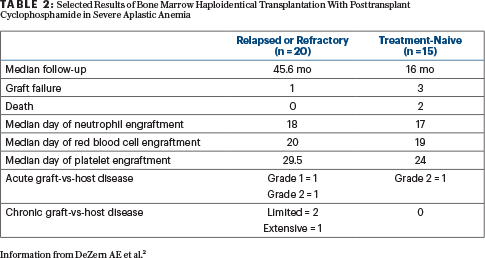
Clinical Implications: Alternative donor bone marrow transplantation (including haploidentical marrow grafts) should be offered to all patients who have relapsed or refractory severe aplastic anemia and who are fit enough to tolerate nonmyeloablative conditioning allogeneic transplant. This approach does open up the option for using allo-HCT using haploidentical donors in treatment-naive severe aplastic anemia, which could lead to a paradigm shift in the field, such that essentially all children and “fit” adults may proceed quickly to a relatively safe, curative transplant approach.
ABSTRACT 697: Excellent overall survival and low incidence of late effects in patients undergoing allo-HCT for sickle cell disease (n = 355): A report from the Center for International Blood and Marrow Transplant Research (CIBMTR)3
Background: Allo-HCT is a curative therapy for sickle cell disease. Good outcomes have spurred an increase in the use of allo-HCT for sickle cell disease, including an increasing number of trials using alternative donors. As survival improves, assessment of long-term outcomes and potential late effects in this patient population is critical.
Methods: Patients reported to the CIBMTR with a diagnosis of sickle cell disease who had undergone their first HCT between 1996 and June 2015 were included in this analysis; patients with graft failure were excluded. The median age at transplant was 10 years (range < 1 to 52 years), and the median follow-up was 50 months (range, 9–248 months).
Results: The most common indications for allo-HCT were recurrent pain (30%), stroke (29%), and acute chest syndrome (13%). The majority of patients received myeloablative conditioning (66%), had an HLA-matched sibling donor (59%), and had bone marrow as the graft source (70%).
• The overall survival rates at 2, 5, and 7 years were 91%, 89%, and 89%, respectively.
• The most common causes of death were organ failure (36%), graft-vs-host disease (18%), and infection (13%). Chronic graft-vs-host disease developed in 30% of patients.
• In multivariable analysis, undergoing transplant after age 15 years was associated with inferior overall survival (mortality hazard ratio [HR] = 7.26, 95% confidence interval [CI] = 3.57–14.77).
• Multivariable analysis for late effects demonstrated an increased risk of diabetes (HR = 7.29, 95% CI = 2.94–18.08) and pulmonary abnormalities (HR = 5.90, 95% CI = 1.14–30.42) with an unrelated donor, whereas older age at the time of transplant was associated with avascular necrosis (HR = 14.19, 95% CI = 1.86–108.47), diabetes (HR = 3.45, 95% CI = 1.69–7.05), and congestive heart failure (HR = 8.02, 95% CI = 1.13–56.94).
• Sickle cell disease–related symptoms overall decreased from pre- to posttransplant, most notably acute chest syndrome, vaso-occlusive pain crises, and stroke.
• New hematologic cancers were diagnosed in four patients after transplantation.
Clinical Implications: Patients with sickle cell disease have an excellent overall survival with a diminished sickle cell disease–related symptom burden after allo-HCT. However, they remain at risk for a myriad of late effects, with numerous barriers to a curative approach of allo-HCT. Although many high-risk patients are potentially cured of their disease, close surveillance for late complications and preventive health maintenance are important aspects of posttransplant follow-up for these patients.
ABSTRACT 872: KD025 for patients with chronic graft-vs-host disease (n = 54): Long-term follow-up of a phase IIa study (KD025-208; ClinicalTrials.gov identifier NCT03640481)4
Background: Chronic graft-vs-host disease exhibits both autoimmune and fibrotic features across multiple organ systems and remains a serious complication after allo-HCT. KD025 is an orally available, Rho-associated, coiled-coil kinase 2 (ROCK2) selective inhibitor that (1) decreases human T cell cytokines interleukin-21 (IL-21) and IL-17 secretion; (2) increases percentages and function of Foxp3+ T-regulatory cells; and (3) reverses established chronic graft-vs-host disease in two distinct preclinical models. KD025 modulates the immune system by shifting the T-helper 17/T-regulatory balance toward homeostasis.
“Chronic graft-vs-host disease exhibits both autoimmune and fibrotic features across multiple organ systems and remains a serious complication after allo-HCT.”— Syed Ali Abutalib, MD, and Navneet Majhail, MD, MS
Tweet this quote
Methods: The primary study endpoint was overall response rate, according to the 2014 National Institutes of Health response criteria in a modified intention-to-treat population. KD025-208 enrolled three sequential cohorts of patients with chronic GvHD after one to three prior lines of systemic therapy: C1: 200 mg daily [n = 17]); C2: 200 mg twice a day [n = 16]; and C3: 400 mg daily [n = 21]). Treatment was in 28-day continuous cycles until disease progression or unacceptable toxicity.
Results: About 63% of patients were refractory to their last line of therapy prior to enrollment, and 50% of patients had chronic graft-vs-host disease in at least four organs. The median duration of treatment was 37, 33, and 39 weeks, in cohorts 1, 2, and 3, respectively. As of June 30, 2019, 24% of patients had received at least 18 months of KD025 therapy. Reasons for treatment discontinuation included chronic graft-vs-host disease progression (n = 18), voluntary withdrawal (n = 7), relapse of underlying disease (n = 5), investigator decision (n = 5), adverse event (n = 3), and death (n = 2).
The overall response rate was 65% (95% CI = 51%–77%) across all three cohorts. Complete responses were observed in all affected organs except the lungs, where partial responses were observed. Responses were durable, with a median duration of response of 34 weeks across all cohorts. Responses were rapid, and often achieved within 8 weeks, although 4 of 35 responses occurred after 24 weeks. During treatment with KD025, the median corticosteroid dose was reduced by 50%. Serious adverse events were reported in 43%, although none were considered related to KD025. (Of note, patients were also on concurrent steroids before the start of the experimental drug.) Three patients discontinued KD025 due to possibly related adverse events (cohort 1: diarrhea, headache; cohort 3: fatigue).
Clinical Implications: According to the investigators, durable and clinically meaningful responses have been observed across all three dosing schedules. The novel ROCK2 inhibitor met its primary endpoint of overall response rate in chronic graft-vs-host disease. KD025 was reported to be well tolerated, and side effects were primarily related to concurrent use of corticosteroids, which was successfully tapered in about 50% of the patients with chronic graft-vs-host disease. The U.S. Food and Drug Administration has granted Breakthrough Therapy designation to KD025 for the treatment of patients with chronic graft-vs-host disease after failure of two or more lines of systemic therapy.
Autologous Hematopoietic Cell Transplantation
ABSTRACT 785: Addition of rituximab to BEAM (carmustine, etoposide, cytarabine, melphalan) conditioning does not improve outcomes of patients with diffuse large B-cell lymphoma (DLBCL) undergoing autologous hematopoietic cell transplantation (auto-HCT): A CIBMTR analysis (n = 862)5
Background: Rituximab-based high-dose therapy with auto-HCT rescue is frequently prescribed to patients with relapsed DLBCL and chemosensitive disease. However, data supporting the benefit of adding rituximab (“in vivo purging”) to auto-HCT conditioning are not available.
Methods: The study cohort was divided into two groups: BEAM (n = 667) and rituximab-BEAM (n = 195). The median follow-up of survivors was 48 months (range, 1–171 months) and 64 months (range, 3–142 months) in the BEAM and rituximab-BEAM cohorts, respectively.
Results: On univariate analysis, the 4-year cumulative incidence of relapse (41% vs 44%), nonrelapse mortality (11% vs 9%), progression-free survival (48% vs 47%), and overall survival (58% vs 61%) were similar in the rituximab-BEAM and BEAM groups, respectively. On multivariate analysis, no significant difference was seen in overall survival (HR = 0.81; 95% CI = 0.81–1.31; P = .83) or progression-free survival (HR = 0.94; 95% CI = 0.76–1.18; P = .61) between the two cohorts. The addition of rituximab had no impact on the risk of relapse (HR = 0.83; 95% CI = 0.65–1.07; P = .15) or nonrelapse mortality (HR = 1.43; 95% CI = 0.909–2.26; P = .12).
Clinical Implications: CIBMTR data show no benefit with the addition of rituximab to BEAM in chemosensitive patients with relapsed DLBCL who are undergoing autologous transplantation.
DISCLOSURE: Dr. Abutalib has served on the advisory board for AstraZeneca and Partner Therapeutics. Dr. Majhail has served as consultant for Anthem, Inc, has served on advisory boards for Incyte and Nkarta, and has received honoraria from Mallinckrodt.
REFERENCES
1. Murdock HM, Kim HT, Hambley B, et al: Genetic alterations at diagnosis predict outcome of AML patients age 60 or older undergoing allogeneic transplantation in first remission. 2019 ASH Annual Meeting & Exposition. Abstract 48. Presented December 7, 2019.
2. DeZern AE, Zahurak M, Cooke KR, et al: The path to cure: Using haploidentical donors and high-dose posttransplant cyclophosphamide for treatment-naive and refractory severe aplastic anemia. 2019 ASH Annual Meeting & Exposition. Abstract 147. Presented December 7, 2019.
3. Stenger E, Phelan R, Shaw BE, et al: Excellent overall survival and low incidence of late effects in patients undergoing allogeneic hematopoietic cell transplant for sickle cell disease: A report from the Center for International Blood and Marrow Transplant Research. 2019 ASH Annual Meeting & Exposition. Abstract 697. Presented December 9, 2019.
4. Jagasia M, Salhotra A, Bachier CR, et al: KD025 for patients with chronic graft-vs-host disease: Long-term follow-up of a phase IIa study. 2019 ASH Annual Meeting & Exposition. Abstract 872. Presented December 9, 2019.
5. Jagadeesh D, Majhail NS, Yizeng H, et al: Does addition of rituximab to BEAM conditioning improve outcomes of patients with diffuse large B-cell lymphoma undergoing autologous hematopoietic cell transplantation? 2019 ASH Annual Meeting & Exposition. Abstract 785. Presented December 9, 2019.

