TO ADD to our ongoing coverage of the 2018 American Society of Hematology (ASH) Annual Meeting & Exposition, we bring readers of The ASCO Post these summaries of an assortment of interesting studies. They focus on novel therapies under investigation in the treatment of acute lymphoblastic leukemia (ALL), myelodysplastic syndromes (MDS), multiple myeloma, diffuse large B-cell lymphoma (DLBCL), and mantle cell lymphoma (MCL).
ALL: Stem Cell Transplant After CAR T-Cell Therapy
UNDERGOING HEMATOPOIETIC stem cell transplant (HSCT) for the first time after CD19 chimeric antigen receptor (CAR) T-cell therapy reduced the risk of recurrence in patients with ALL, but a second HSCT after CD19 CAR T-cell therapy did not improve leukemia-free survival.1 These results of a retrospective analysis of the phase I/II PLAT-02 study suggest that HSCT can reduce the risk of relapse only in patients who have not had a prior HSCT before CAR T-cell therapy.

Corinne Summers, MD
“Although these numbers of subjects are small, the data suggest a leukemia-free survival benefit for those without a history of transplant who pursued transplant once in remission,” stated the principal investigator Corinne Summers, MD, of Ben Towne Center at Seattle Children’s Hospital, Washington. “The role of HSCT after CD19 CAR T-cell therapy for patients who have a history of transplant is unclear, and larger studies are needed.”
PLAT-02 included 64 pediatric and young adults with CD19-positive B-cell ALL. In phase I, CAR T-cell therapy given at 4 different dose levels achieved robust responses in 43 patients, with a minimal residual disease (MRD)-negative complete remission rate of 93% 21 days after treatment. In phase II, 21 patients were treated with the second lowest dose of CAR T-cell therapy, which seemed to achieve longer responses in CAR T-cell persistence.
The rate of 1-year leukemia-free survival was 76%. Late relapses continue to occur, Dr. Summers noted, including CD19-positive relapses at 22 and 27 months as well as CD19-negative and myeloid relapses at 41 and 37 months, respectively, after CAR T-cell infusion.
At Seattle Children’s Hospital, where this study was conducted, patients with relapsed or refractory ALL and no prior transplant typically undergo HSCT once in remission. “Although remission is frequently attained, approximately half of all patients recur. However, the benefit of HSCT after CD19 CAR T-cell therapy is controversial,” Dr. Summers said.
“The data suggest a leukemia-free survival benefit [with HSCT after CD19 CAR T-cell therapy] for those without a history of transplant who pursued transplant once in remission.”— Corinne Summers, MD
Tweet this quote
The researchers conducted a retrospective analysis of study patients who sustained leukemic remission for more than 63 days after CAR T-cell therapy. The analysis looked at 32 patients from the phase I portion of the trial and 18 patients from the initial phase II cohort, who were followed for at least 1 year.
Of 50 evaluable patients, 34 had received at least a single prior HSCT and 16 had not. A trend was observed for improved leukemia-free survival among all patients who underwent HSCT after CAR T-cell therapy, but there was no survival benefit in either group.
Thirteen patients with no history of HSCT underwent HSCT after CAR T-cell infusion; 1 patient relapsed, whereas 2 of the 3 who did not undergo HSCT after CAR T-cell therapy relapsed. A third patient is in remission at 28 months of follow-up.
Of patients who had a previous HSCT, 24 did not undergo a second HSCT after CAR T-cell therapy; 7 remain in remission. Of the 10 patients who had a second HSCT, 5 remain in remission after at least 24 months of follow-up.
MDS: Immune Checkpoint Inhibition
IN PATIENTS with MDS, immune checkpoint inhibition plus azacitidine may prove to be an active therapy, in a study reported by Guillermo Garcia-Manero, MD, of The University of Texas MD Cancer Center.2

Guillermo Garcia-Manero, MD
Myeloid cells express programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), and their expression is activated by treatment with hypomethylating agents. The study’s hypothesis was that in patients with MDS, immune checkpoint inhibitors that block CTLA or PD-1, with or without azacitidine, may be active both in front-line and relapsed disease.
“We showed the incorporation of immune checkpoint inhibitors in MDS is feasible; there is single-agent activity of ipilimumab after hypomethylating agent failure; and we can achieve a high response rate of 70% (included complete responses in 40%) with nivolumab plus azacitidine in the front-line setting. We also saw that the median overall survival was not reached with the combination of ipilimumab and azacitidine,” Dr. Garcia-Manero said.
“We saw that the median overall survival was not reached with the combination of ipilimumab and azacitidine.”— Guillermo Garcia- Manero, MD
Tweet this quote
Findings came from a “basket” exploratory phase II trial of patients with MDS, both untreated (front-line cohort) and those whose disease progressed on hypomethylating agents (hypomethylating agent failure cohort). In the front-line setting, two regimens were tested: azacitidine plus nivolumab (n = 20) and azacitidine plus ipilimumab (n = 21). Two treatments were also tested in the hypomethylating agent failure cohort: single-agent nivolumab (n = 15) and single-agent ipilimumab (n = 20); if there was no response to the checkpoint inhibitor after 6 cycles (or less), they could also receive azacitidine, to test the concept of resensitization.
Nivolumab was administered at 3 mg/kg on days 1 and 15 every 4 weeks and ipilimumab, at 3 mg/kg on day 1 every 3 weeks. Azacitidine was used at the standard dose. When combined with azacitidine, nivolumab was administered on days 6 and 20 every 4-week cycle, and ipilimumab was given on day 6 every 4 weeks.
High response rates were observed in the front-line setting (Table 1), with activity also seen with single-agent ipilimumab after hypomethylating agent failure, Dr. Garcia-Manero reported.
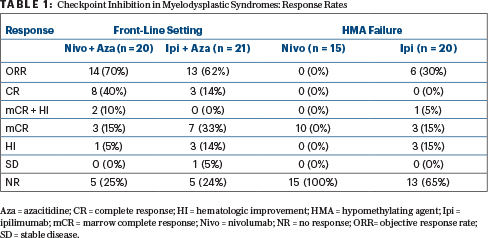
In newly diagnosed MDS, after 20 months of median follow-up, the median overall survival was not reached with azacitidine/ ipilimumab and was 11.8 months with azacitidine/nivolumab. As for survival, 1-year survival rates were 68% and 50%, respectively. After hypomethylating agent failure, the median survival was 8.5 months with ipilimumab and 8.0 months with nivolumab. There was some indication that PD-1 expression corresponded with response, he reported.
Multiple Myeloma: Oral Selinexor With Low-Dose Dexamethasone
IN PATIENTS with pentarefractory multiple myeloma in the STORM trial, the oral XPO1 inhibitor selinexor, in combination with low-dose dexamethasone, induced an overall response rate of 26.2%, with 39.3% of patients deriving a clinical benefit and 79% achieving stable disease.3 The median progression-free survival was 3.7 months, and the median overall survival was 8.6 months.
Thus, this oral XPO1 inhibitor is the first investigational oral therapy to show activity in patients refractory to at least one proteasome inhibitor, at least one immunomodulatory drug, daratumumab, and glucocorticoid, according to Ajai Chari, MD, of Mount Sinai School of Medicine, New York.

Ajai Chari, MD
“STORM Part 2 represents the largest, most heavily pretreated population with myeloma in a prospective clinical trial to date,” he said. Patients received a median of 7 prior therapies over 6.6 years and had no other viable treatment options.
Dr. Chari noted that persons with myeloma are increasingly being treated with numerous drugs: the proteasome inhibitors bortezomib and carfilzomib; the immunomodulatory drugs lenalidomide and pomalidomide; and the anti-CD38 monoclonal antibody daratumumab. “Eventually, patients develop pentaexposed myeloma and myeloma that is refractory to all of these classes,” Dr. Chari said. “Their prognosis is dismal, with a median overall survival as short as 1.3 to 3.5 months. There are no approved drugs with established clinical activity in triple class–refractory myeloma.”
The analysis included 123 patients with pentaexposed and triple class–refractory disease treated with 80 mg of selinexor plus 20 mg of dexamethasone twice weekly in 28-day cycles. About half the patients had high-risk cytogenetics; 68% had pentarefractory myeloma; 84% had a prior stem cell transplant; almost 30% had received at least 9 prior lines of therapy; and 96% were refractory to carfilzomib, pomalidomide, and daratumumab.
The 26.2% response rate included 19.7% with a very good partial response and 6.5% with a very good partial response or better. In patients with progressive disease as the best response, the median overall survival was 1.7 months; this survival improved to 5.9 months in those who achieved stable disease and to 15.6 months in responders.
Nausea, fatigue, anorexia, and weight loss (any grades) were seen in approximately half of patients, and about one-third of patients experienced hyponatremia, vomiting, and diarrhea. Grade ≥ 3 toxicities were mostly fatigue (18.7%), hyponatremia (16.3%), and nausea (9.8%). The most common treatment-related hematologic toxicities were thrombocytopenia and anemia; grade 3 or 4 thrombocytopenia increased as baseline platelet counts decreased. “Side effects were reversible without evidence of major organ toxicities nor cumulative toxicity,” Dr. Chari reported.
DLBCL: Long-Term Follow-up With Axicabtagene Ciloleucel
LONG-TERM follow-up of the ZUMA-1 trial showed that the CAR T-cell therapy axicabtagene ciloleucel continued to achieve durable responses and survival benefits.4 The 2-year overall survival rate was 51% for patients with refractory large B-cell lymphoma treated with axicabtagene ciloleucel. These results led to the U.S. Food and Drug Administration (FDA) approval of axicabtagene ciloleucel.
At a median follow-up of 27.1 months after axicabtagene ciloleucel therapy, the 24-month progression-free survival rate was 39%, and the median progression-free survival was 5.9 months. A total of 39% of patients had an ongoing response, and the median duration of response was not reached for those in complete remission. The mean overall survival was not yet reached.
These findings were published online in The Lancet Oncology to coincide with the presentation at the 2018 ASH Annual Meeting & Exposition.5 The lead investigator was Sattva Neelapu, MD, of The University of Texas MD Anderson Cancer Center, Houston.

Sattva Neelapu, MD
ZUMA-1 included 108 patients: 101 from the phase II portion and 7 from the phase I portion. Phase II included 77 patients with DLBCL and 24 patients with primary mediastinal large B-cell lymphoma, and DLBCL transformed from follicular lymphoma.
In the primary results of the trial, the objective response rate was 83% according to investigator assessment and 74% by central review. The complete response rates were 58% and 54%, respectively. At the time of the 2018 ASH presentation, the updated analysis found that 39% and 36% of patients, respectively, continued to respond to treatment. In 37 patients with double-expressor B-cell lymphoma, 91% responded, including complete responses in 70%. Overall, 48% continued to respond at the time of the updated analysis.
CAR T cells persisted over time in patients with ongoing responses. In the updated analysis, there were no new cases of cytokine-release syndrome nor neurotoxicity. Treatment-emergent cytopenias ≥ grade 3 occurred in 86% of patients.
MCL: BTK Inhibitors Acalabrutinib and Zanubrutinib
THE BRUTON’S tyrosine kinase (BTK) inhibitor acalabrutinib has been approved by the FDA for the treatment of relapsed or refractory MCL, based on data from a single-arm, multicenter phase II trial called ACE-LY-004. Longer-term follow-up of this study demonstrated durable responses and no new safety signals in patients with relapsed or refractory disease treated with acalabrutinib.6
At a median follow-up of 26 months, 49 of 124 patients (40%) remain on treatment. The investigator-assessed response rate was 81%; which was identical to the 15-month response rate previously reported.7 The complete response rate increased from 40% at 15 months to 43% at 26 months. Consistent responses were observed among all subgroups.
“The responses stayed the same [as the previously published data7], and the tolerability of this drug is amazing,” said lead author Michael Wang, MD, Director of the MCL Program at The University of Texas MD Anderson Cancer Center. “This is a single drug with durable response. Its efficacy and tolerability stand the test of time.”

Michael Wang, MD
ACE-LY-004 enrolled 124 patients with relapsed or refractory MCL and treated them with oral acalabrutinib at 100 mg twice daily in 28-day cycles. Forty-four percent were characterized as intermediate risk; 8% had bulky lymph nodes; 72% had extranodal disease; and 24% were refractory to their last treatment, which included transplant in 18% (none received a BTK inhibitor). The median number of prior treatments was two.
The median duration of response was 36 months. The median progression-free survival was 20 months; the 24-month progression-free survival was 49%; and the estimated 24-month overall survival was 72%. During the study, 43 people died: 23% due to progressive disease and 5% due to adverse events not deemed related to treatment.
“[Acalabrutinib] is a single drug with durable response. Its efficacy and tolerability stand the test of time.”— Michael Wang, MD
Tweet this quote
Zanubrutinib also showed activity in 86 adult patients with relapsed or refractory MCL treated in a phase II trial in China.8 This investigational BTK inhibitor achieved a response rate of 83.5% as well as a progression-free survival rate of 90% at 12 weeks and 82% at 24 weeks. At a median follow-up of 35.9 weeks, the median progression-free survival was not reached.
“Zanubrutinib achieves high rates of response and complete response, as documented by [positron-emission tomography] activity. The responses achieved with zanubrutinib treatment appear to be durable, although we need longer follow-up time to confirm these results,” said Yuqin Song, MD, of Peking University Cancer Hospital, China.

Yuqin Song, MD
Patients had received from one to four prior regimens. They received zanubrutinib at 160 mg twice daily until disease progression or unacceptable toxicity. About half of the patients had refractory disease, and the other half relapsed. More than 90% had stage III/IV disease, and 83.7% had intermediate- or high-risk disease, according to the MCL International Prognostic Combined Biologic Index.
A total of 85 patients were evaluable for efficacy. Of the 71 responders, 58.8% had complete responses and 24.7% had partial responses; 2.4% of patients achieved stable disease. The treatment effect of zanubrutinib was consistent across all subgroups, and the drug’s tolerability was consistent with previous studies of this agent in B-cell malignancies.
Zanubrutinib was granted Fast Track designation by the FDA in the treatment of Waldenström’s macroglobulinemia. Data from the phase II trial reported by Dr. Song, along with data from a phase I trial in relapsed or refractory B-cell non-Hodgkin lymphoma, are included in a new drug application submission in China for zanubrutinib in relapsed or refractory MCL. The drug is also being studied in relapsed or refractory chronic lymphocytic leukemia and large B-cell lymphoma.
Microbiota Diversity: Predicting Outcomes After Transplant
IN PATIENTS undergoing allogeneic stem cell transplantation, a diverse microbiome before transplant seemed to be associated with better survival and fewer complications, in an international study reported by Jonathan U. Peled, MD, PhD, of Memorial Sloan Kettering Cancer Center, New York.9

Jonathan U. Peled, MD, PhD
Dr. Peled and his colleagues examined 1,922 stool samples from 991 allogeneic transplant recipients at 4 international transplant centers on 3 continents. The underlying diagnosis varied, as did the donor-graft source, conditioning intensity, and prophylaxis for graft-vs-host disease. The average patient demonstrated less microbiota diversity than healthy controls (1.7- to 2.5-fold lower; P < .001). The microbiota configurations for the patients were similar across all institutions.
Severe microbiota injury (as revealed by domination—where a single organism comprised more than 30% of bacteria in the gut) was common and predictive of worse overall survival. At all the centers, up to 87% of patients ultimately demonstrated intestinal domination, with the dominating taxa being primarily Enterococcus, Streptococcus, Lactobacillus, Escherichia, and Klebsiella. The authors hypothesized that a low-diversity state is associated with exposure to broad-spectrum antibiotics, intensive conditioning, and low caloric intake.
The pretransplant period may offer a “window of opportunity” to assess microbiota injury and to intervene, thus establishing a more favorable environment and increasing the chance for better outcomes, Dr. Peled suggested. “If we can come up with a way to remediate microbiome injury, there might be time to implement it prior to transplant.” ■
DISCLOSURE: Dr. Summers has received financial support from Juno Therapeutics, Inc. Dr. Garcia-Manero has received research support from Bristol-Myers Squibb, Astex, Celgene, Merck, and Novartis and is a consultant for Celgene and Astex. Dr. Chari is a consultant, advisor, or on the Board of Directors for Novartis, Adaptive Biotechnology, The Binding Site, Seattle Genetics, Celgene, Amgen, Takeda, Janssen, Bristol-Myers Squibb; and has received research funding from Novartis, Array Biopharma, Celgene, Amgen, Pharmacyclics, Takeda, and Janssen. Dr. Neelapu is a consultant/advisor for Merck Sharp & Dohme, Celgene, Kite Pharma, Novartis, and Unum Therapeutics; has received research funding from Celgene, Bristol-Myers Squibb, Merck Sharp & Dohme, Kite Pharma, Pharmacyclics, Acerta Pharma, Cellectis, and Poseida Therapeutics; and travel/accommodations/expenses from Dava Oncology. Dr. Wang has received honoraria from Celgene, Dava Oncology, Pharmacyclics, Janssen, Acerta Pharma; research funding from Celgene, AstraZeneca, Pharmacyclics, Janssen, Juno, Acerta Pharma, Kite Pharma, Novartis, and BeiGene; is a consultant for AstraZeneca, Janssen, Pulse Biosciences, and MoreHealth; and is on the Board of Directors or an advisor for Celgene, Janssen, and AstraZeneca. Dr. Song’s study was supported by BeiGene, and she reported no conflicts of interest. Dr. Peled has financial relationships with Seres Therapeutics, Parker Institute for Cancer Immunotherapy, and Merck/Society for Immunotherapy of Cancer.
REFERENCES
1. Summers C, Annesley C, Bleakley M, et al: Long-term follow-up after SCRI-CAR19v reveals late recurrences as well as a survival advantage to consolidation with HCT after CAR T cell induced remission. 2018 ASH Annual Meeting & Exposition. Abstract 967. Presented December 3, 2018.
2. Garcia-Manero G, Sasaki K, Montalban-Bravo G, et al: A phase II study of nivolumab or ipilimumab with or without azacitidine for patients with myelodysplastic syndrome. 2018 ASH Annual Meeting & Exposition. Abstract 465. Presented December 2, 2018.
3. Chari A, Vogl DT, Dimopoulos MA, et al: Results of the pivotal STORM Study (Part 2) in penta-refractory multiple myeloma (MM): Deep and durable responses with oral selinexor plus low dose dexamethasone in patients with penta exposed and triple-class refractor MM. 2018 ASH Annual Meeting & Exposition. Abstract 598. Presented December 3, 2018.
4. Neelapu SS, Ghobadi A, Jacobson CA, et al: 2-Year follow-up and high-risk subset analysis of Zuma-1, the pivotal study of axicabtagene ciloleucel in patients with refractory large B-cell lymphoma. 2018 ASH Annual Meeting & Exposition. Abstract 2967. Presented December 2, 2018.
5. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol 20:31-42, 2019.
6. Wang M, Rule S, Zinzani PL, et al: Long-term follow-up of acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma. 2018 ASH Annual Meeting & Exposition. Abstract 2876. December 2, 2018.
7. Wang M, Rule S, Zinzani PL, et al: Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): A single-arm, multicentre, phase 2 trial. Lancet 391:659-667, 2018.
8. Song Y, Zhou K, Zou D, et al: Safety and activity of the investigational Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) in patients with mantle cell lymphoma from a phase 2 trial. 2018 ASH Annual Meeting & Exposition. Abstract 148. Presented December 1, 2018.
9. Peled JU, Gomes ALC, Stein-Thoeringer CK, et al: Multicenter microbiota analysis indicates that pre-HCT microbiota injury is prevalent across geography and predicts poor overall survival. 2018 ASH Annual Meeting & Exposition. Abstract 811. Presented December 3, 2018.

