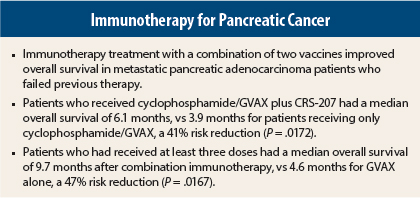Overall survival was improved in metastatic pancreatic cancer patients through an innovative immunotherapy strategy in a multicenter study reported at the 2014 Gastrointestinal Cancers Symposium.1
“This is the first time a randomized study has shown that immunotherapy is effective in pancreatic cancer,” said Dung T. Le, MD, Assistant Professor of Medicine at the Johns Hopkins University Sidney Kimmel Comprehensive Cancer Center, Baltimore. “This is just a first step, and we believe we’ll be able to take this approach further.”
How the Treatment Works
The novel treatment, which may be better tolerated than standard chemotherapy, involves two different anticancer vaccines: GVAX Pancreas followed by CRS-207. GVAX is composed of pancreatic cancer cells that have been genetically modified to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF), which stimulates the immune system. GVAX is given with low-dose cyclophosphamide to inhibit regulatory T cells and boost the vaccine’s efficacy. The second vaccine, CRS-207, is live-attenuated Listeria monocytogenes (Lm), which has been genetically modified to be safe for human use while retaining its ability to stimulate an immune response against the protein mesothelin on pancreatic tumor cells.
The combination essentially trains the body to recognize and attack pancreatic tumors. In mouse tumor models, Lm/GVAX vaccines are synergistic, and in a phase I study of CRS-207, patients with pancreatic ductal adenocarcinoma who had received prior GVAX lived more than 15 months, Dr. Le explained.
Study Details
The study randomly assigned 90 pancreatic adenocarcinoma patients 2:1 (51% with two or more prior treatments and 83% with one prior treatment for metastatic disease) to two doses of GVAX followed by four doses of CRS-207, or six doses of GVAX alone, every 3 weeks. (Low-dose cyclophosphamide was always given the day before GVAX.) Courses could be repeated. The primary endpoint was overall survival.
At a planned interim analysis, and at a median follow-up of 7.8 months, in the population of patients who received at least one dose of the vaccine, median overall survival was 6.1 months with the combination vs 3.9 months with GVAX alone, a 41% reduction in risk with the combination immunotherapy (P = .0172).
The greatest differences were observed in patients who received three total doses, which includes at least two doses of GVAX and at least one dose of CRS-207. Median overall survival in this population was 9.7 months, vs 4.6 months for GVAX alone, a 47% reduction (P = .0167), she reported.
A striking difference was also seen in the subgroup of patients who had had two or more prior chemotherapy regimens. Receipt of combination immunotherapy in the third line or greater led to a median overall survival of 5.7 months, vs 3.7 with GVAX, a 70% reduction in risk that was highly significant (P = .0003). In the per-protocol group (who received at least three doses), median overall survival for this subgroup was 8.3 months vs 4.0 months, respectively, a 68% reduction in risk (P = .0002), she added.
There were no responses, but stable disease was observed in 37% of the combination group and 30% of the GVAX-only group. CA19-9 stabilization was observed in 32% receiving the combination, vs 13% of the single-agent group (P = .06). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration.
The 1-year survival probability was estimated at 24% for the combination arm and 12% in the GVAX-only arm.
“The vaccines appear to be safe and well tolerated,” Dr. Le added. A three-arm study is evaluating the combination compared to CRS-207 alone and to chemotherapy. ■
Disclosure: Dr. Le reported no potential conflicts of interest.
Reference
1. Le DT, Wang-Gillam A, Picozzi V Jr, et al: A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. 2014 Gastrointestinal Cancers Symposium. Abstract 177. Presented January 17, 2014.