
Local treatment of breast cancer is trending toward less invasive procedures that achieve comparable outcomes to standard interventions. What will the next step along this continuum be? According to Michael S. Sabel, MD, a surgical oncologist at the University of Michigan Comprehensive Cancer Center, Ann Arbor, “In situ ablation of small breast cancer tumors is the future of breast cancer.”
Dr. Sabel described this emerging, though still unproven, modality during the “Debates in Surgical Oncology” session at the 97th Annual American College of Surgeons Clinical Congress in San Francisco. Surgeons in attendance agreed, with 73% predicting (via an audience response system) that ablation will become an option for a small subset of women eligible for breast-conserving therapy within 10 years.
“The techniques are already routine in some cancers, so there is a certain degree of inevitability about this,” he commented. Indeed, some “ablation centers” are already trolling for patients, but this is jumping the gun, he acknowledged.
“We need to resist the urge to adopt an unproven therapy, but we don’t want to discard a potentially promising one,” Dr. Sabel said.
Three Techniques
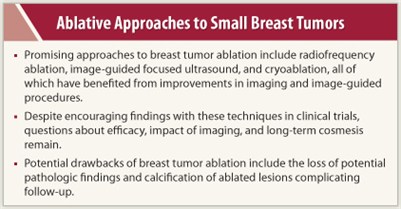
The most promising approaches are radiofrequency ablation, image-guided focused ultrasound, and cryoablation. Improvements in imaging and image-guided procedures have made these novel approaches feasible.
Radiofrequency ablation generates heat via an electrical current that produces high-frequency vibrations of electrons. Probes are deployed into the tumor, target temperatures are achieved within 15 minutes, and the result is a 3- to 5-cm diameter lesion. Replacing lumpectomy with in situ ablation could conceivably reduce the complexity and cost of care and significantly improve cosmesis. But due to its complexity and its potential for complications, radiofrequency ablation has not gained favor as a primary method of ablating tumor.
 Recently, investigators used excision followed by radiofrequency ablation in an attempt to ensure eradication of micrometastases, replacing the role of radiation therapy. In a study of 41 patients (average tumor size 1.6 cm, 25% with inadequate margins), lumpectomy followed by radiofrequency ablation allowed 24 patients to avoid radiation therapy, and it reduced the need for re-excision by 91%. At a median follow-up of 24 months, there were no local recurrences in this study by Klimberg et al.1
Recently, investigators used excision followed by radiofrequency ablation in an attempt to ensure eradication of micrometastases, replacing the role of radiation therapy. In a study of 41 patients (average tumor size 1.6 cm, 25% with inadequate margins), lumpectomy followed by radiofrequency ablation allowed 24 patients to avoid radiation therapy, and it reduced the need for re-excision by 91%. At a median follow-up of 24 months, there were no local recurrences in this study by Klimberg et al.1
The multicenter ABLATE trial will evaluate lumpectomy followed by radiofrequency ablation as a way to extend the “final” negative margin and decrease rates of reoperation and local recurrence. The next evolution of this strategy is to combine it with percutaneous excision, an even less invasive method than lumpectomy plus radiofrequency ablation. In a recent study of percutaneous excision followed by radiofrequency ablation, all 15 patients demonstrated 100% ablation and clear margins.2
Focused Ultrasound Ablation and Cryoablation
An even less invasive approach—no surgery is involved—is focused ultrasound ablation. When the procedure was performed under MRI guidance, no local recurrences were seen among 47 patients followed for 43 months in a Japanese study.3 “The images seen in that study exemplify the cosmetic potential of ablative technologies over lumpectomy,” Dr. Sabel said.
The American College of Radiology Imaging Network (ACRIN) will soon evaluate MRI-guided focused ultrasound ablation followed by excision in the ACRIN 6674 trial.
Dr. Sabel has been researching cryoablation, and in his feasibility study of 29 patients, the approach was highly effective for tumors of any histology < 1.0 cm and for ductal cancers without an extensive in situ component < 1.5 cm.4
Such encouraging findings led to a phase II trial, American College of Surgeons Oncology Group (ACOSOG) Z1072, in which cryoablation will be followed by lumpectomy, with pre- and post-treatment MRI to determine residual disease. The trial will also assess the power of cryoablation to stimulate a systemic antitumor immune response.
Unresolved issues include questions of efficacy (long-term local recurrence rates), impact of imaging (ability to detect local recurrence after ablation), and long-term cosmesis (lesions may persist, which may worry patients and physicians).
“We need long-term follow-up evidence of good local control, and this requires well organized trials,” he said.
Others Are Doubtful
 Not all surgeons view ablation as promising. “I am concerned that you lose the pathologic details when you don’t take out the tumor and view it under the microscope. We don’t know how this might affect overall treatment planning; there is a potential for impact beyond just the local control,” Seema A. Khan, MD, Professor of Surgery and the Bluhm Family Professor of Cancer Research at Northwestern University Feinberg School of Medicine, Chicago, told The ASCO Post.
Not all surgeons view ablation as promising. “I am concerned that you lose the pathologic details when you don’t take out the tumor and view it under the microscope. We don’t know how this might affect overall treatment planning; there is a potential for impact beyond just the local control,” Seema A. Khan, MD, Professor of Surgery and the Bluhm Family Professor of Cancer Research at Northwestern University Feinberg School of Medicine, Chicago, told The ASCO Post.
“With surgery, we already achieve good cosmesis. But some of the ablated lesions calcify and become hard to follow. Ablation needs careful study, and the details need to be worked out. In addition, treatment times with some of the ablation techniques can be quite long. At this point, I am skeptical, but open to persuasion with data,” she said. ■
Disclosure: Drs. Sabel and Khan reported no potential conflicts of interest.
References
1. Klimberg VS, Kepple J, Shafirstein G, et al: eRFA: Excision followed by RFA—a new technique to improve local control in breast cancer. Ann Surg Oncol 13:1422-1433, 2006.
2. Klimberg VS, Boneti C, Adkins LL, et al: Feasibility of percutaneous excision followed by ablation for local control in breast cancer. Ann Surg Oncol 18:3079-3087, 2011.
3. Furusawa H: Plenary presentation. 2nd International Symposium on MR-guided Focused Ultrasound. Washington, DC, October 2010.
4. Sabel MS, Kaufman CS, Whitworth P, et al: Cryoablation of early-stage breast cancer: Work-in-progress report of a multi-institutional trial. Ann Surg Oncol 11:147-156, 2004.

