The antibody-drug conjugate sacituzumab govitecan-hziy (sacituzumab govitecan) is beneficial in previously treated patients with metastatic triple-negative breast cancer, irrespective of Trop-2 expression, according to a biomarker analysis of the phase III ASCENT trial.1
Although greater efficacy was observed among patients with medium or high Trop-2 expression, patients of all Trop-2 expression subgroups benefited from treatment with the agent, as compared with physician’s choice of therapy, Sara A. Hurvitz, MD, reported at the 2020 San Antonio Breast Cancer Symposium. Dr. Hurvitz is Professor of Medicine and Director of the Breast Cancer Clinical Research Program at the University of California Los Angeles and Medical Director of the Jonsson Comprehensive Cancer Center Clinical Research Unit.

Sara A. Hurvitz, MD
Sacituzumab govitecan is a first-in-class antibody-drug conjugate that is directed toward Trop-2, an epithelial antigen expressed on many solid tumors. Up to 88% of triple-negative primary and metastatic tumors show moderate-to-strong expression of Trop-2, Dr. Hurvitz said.
Sacituzumab govitecan received accelerated approval for the treatment of metastatic triple-negative breast cancer based on the ASCENT trial. This study showed a significant survival improvement with sacituzumab govitecan over chemotherapy: median 12.1 vs 6.7 months (hazard ratio [HR] = 0.48; P < .0001).2
Findings From Biomarker Analysis
Dr. Hurvitz presented the exploratory biomarker analysis in the brain metastasis–negative population of 468 patients. Assessments included Trop-2 expression and germline BRCA1/2 mutation status. Only patients with known Trop-2 and BRCA1/2 results were included in this analysis.
Trop-2 expression was assessed using a validated assay and categorized based on the H-score, a numerical value (0–300) representing a weighted summation of percent staining. The results were high Trop-2 expression (200–300) seen in about 53% of patients, medium expression (100–200) found in 25%, and low expression (< 100) reported in approximately 20%.
In the high Trop-2 subgroup, the benefit of sacituzumab govitecan was most striking, with a median progression-free survival of 6.9 months vs 2.5 months with standard therapy; the median overall survival in this subgroup was 14.2 months vs 6.9 months. Other subgroups of Trop-2 expression also benefited from the antibody-drug conjugate (Table 1), Dr. Hurvitz reported.

“In terms of overall survival, sacituzumab govitecan outperformed chemotherapy for all subgroups,” she reported. “Median survival was longest in the sacituzumab govitecan–treated group with high or medium Trop-2 expression. For response, sacituzumab govitecan also outperformed chemotherapy across all Trop-2 expression subgroups, with a significant difference between the arms noted for high Trop-2- expression.”
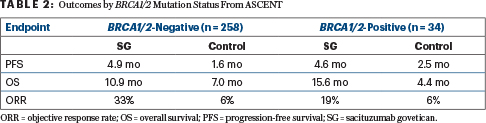
By BRCA1/2 status, sacituzumab govitecan was also superior to physician’s choice of chemotherapy regardless of the presence or absence of mutation (Table 2). However, Dr. Hurvitz noted, the BRCA-positive subset was small (n = 34), and those outcomes should be interpreted with caution.
A similar safety profile was seen across subgroups, showing that Trop-2 expression did not affect toxicity, she added.
DISCLOSURE: Dr. Hurvitz has received funds for research support from Ambrx, Amgen, Arvinas, Bayer, Daiichi Sankyo, Dignitana, Genentech, Gilead, GSK, Immunomedics, Lilly, MacroGenics, Novartis, OBI Pharma, Pfizer, Pieris, Puma Biotechnology, Phoenix Molecular Designs, Radius, Roche, Sanofi, and Seattle Genetics and has stock options in NKMax.
REFERENCES
1. Hurvitz SA, Tolaney SM, Punie K, et al: Biomarker evaluation in the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. 2020 San Antonio Breast Cancer Symposium. Abstract GS3-06. Presented December 10, 2020.
2. Bardia A, Tolaney SM, Loirat D, et al: ASCENT: A randomized phase III study of sacituzumab govitecan vs treatment of physician’s choice in patients with previously treated metastatic triple-negative breast cancer. ESMO Virtual Congress 2020. Abstract LBA17. Presented September 19, 2020.

