TWO IMPORTANT STUDIES, both updates of earlier findings and presented at the 2017 San Antonio Breast Cancer Symposium, provided different findings as to the relative benefit of nanoparticle albumin-bound (nab)-paclitaxel (Abraxane), vs solvent-based paclitaxel in breast cancer.

C. Kent Osborne, MD, FASCO
“The two studies were somewhat different in their results. In one, GeparSepto, nab-paclitaxel looked better. In the other, CALGB 40502, it did not. Where nab-paclitaxel fits into the current treatment armamentarium, I am not sure,” C. Kent Osborne, MD, FASCO, Director of the Dan L. Duncan Cancer Center at Baylor College of Medicine, Houston, told The ASCO Post.
GeparSepto Neoadjuvant Trial
THE PHASE III GeparSepto study was conducted at 69 cancer centers by the German Breast Group. The first survival analysis was presented in San Antonio by Andreas Schneeweiss, MD, of the University of Heidelberg in Germany.1
GeparSepto evaluated neoadjuvant chemotherapy using weekly nab-paclitaxel vs solvent-based paclitaxel, followed by additional chemotherapy, in 1,204 high-risk early breast cancer patients (stages cT2–cT4a-d and cT1 with additional risk factors). The primary endpoint was achievement of a pathologic complete response.

Andreas Schneeweiss, MD
Not only were the rates of pathologic complete response higher with nab-paclitaxel, but this correlated with an improvement in disease-free survival, compared to standard paclitaxel, Dr. Schneeweiss reported.
Patients were randomized to receive either 12 weekly cycles of solvent-based paclitaxel at 80 mg/m2, or 12 weekly cycles of nab-paclitaxel at 150 mg/m2, each followed by 4 cycles of epirubicin/cyclophosphamide (EC). In addition to chemotherapy, all patients received standard adjuvant therapy, and those with HER2-positive disease also received trastuzumab (Herceptin) plus pertuzumab (Perjeta).
At a preplanned safety interim analysis, after 464 patients were enrolled, the dosage of nab-paclitaxel was reduced to 125 mg/ m2 to ameliorate the occurrence of neuropathy associated with the initial dosing. This resulted in a reduction in grade 3/4 neuropathy from 15% to 8%, Dr. Schneeweiss said.
Response and Survival Data
INVESTIGATORS PREVIOUSLY reported that the substitution of nab-paclitaxel for solvent-based paclitaxel significantly increased the rate of pathologic complete response from 29% to 38% (P < .001), but other outcomes had not been determined.2
“It had not yet been shown whether this 9% improvement in pathologic complete response would translate into an improvement in survival,” Dr. Schneeweiss said.
The analysis of disease-free survival occurred after 244 events, at a median follow-up of 49 months, at which time 141 events were noted with standard paclitaxel vs 103 with nab-paclitaxel. This resulted in an absolute improvement in disease-free survival of 6.4% at 3 years (hazard ratio [HR] = 0.69, P = .0044; Table 1).
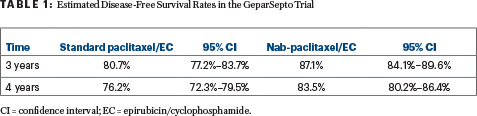
Only 16 patients would need to be treated with nab-paclitaxel to prevent one disease-free survival event within 3 years—“a number that is important because, so far, 61% of the disease-free survival events in GeparSepto have been distant relapses,” he commented.
Nab-paclitaxel’s effect was most “pronounced,” he said, in patients with triple-negative breast cancer, whose 3-year disease-free survival rate was 83.1% with nab-paclitaxel vs 73.4% with standard paclitaxel (HR = 0.66, P = .0694). The benefit was nearly as great for the hormone receptor–positive/HER2-negative subset, of whom 86.3% vs 78.6%, respectively (HR = 0.71, P = .0660), were disease-free at 3 years.
“Probably due to the low number of events, those differences did not reach formal statistical significance; however, there’s a strong trend,” he observed.
The greater improvement with nab-paclitaxel was true for all subgroups, and the interaction test for Ki67 was significant. Patients with either high or low baseline Ki67 derived benefit from nab-paclitaxel, he pointed out.
The overall survival data are not mature, but at 4 years, 89.6% of the nab-paclitaxel arm and 87.0% of the standard paclitaxel arm were alive.
“GeparSepto confirms the surrogate value of pathologic complete response for disease-free survival,” he said. Patients who achieved a pathologic complete response, regardless of whether they received nab-paclitaxel or solvent-based paclitaxel, had an improved and favorable disease-free and overall survival, but those who did not achieve a pathologic complete response had significantly better outcomes if they had received nab-paclitaxel.
“This interesting finding, if it is real, argues for an effect of nab-paclitaxel on disease-free survival beyond its effect on improving pathologic complete responses,” Dr. Schneeweiss suggested.
CALGB 40502/NCCTG N063H
IN AN UPDATE of the CALGB 40502/NCCTG N063H trial, presented in San Antonio by Hope Rugo, MD, FASCO, Director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco, previously untreated metastatic breast cancer patients fared better with standard paclitaxel than with nab-paclitaxel, though nab-paclitaxel was more effective in triple-negative disease.3 The study also confirmed the efficacy of a weekly taxane regimen.

Hope Rugo, MD, FASCO
The phase III trial randomized 799 patients between 2008 and 2011 to receive paclitaxel, nab-paclitaxel, or ixabepilone (Ixempra). Most patients also received concurrent bevacizumab (Avastin), which was approved for breast cancer at the time. Nab-paclitaxel was given at a dose of 150 mg/m2 weekly (per the manufacturer’s request), a high dose that is no longer used. Paclitaxel was administered at 90 mg/ m2 and ixabepilone, at 16 mg/m2 weekly.
In the previously reported primary analysis, neither experimental arm demonstrated superiority to paclitaxel, and ixabepilone proved to be significantly inferior. At this year’s meeting, Dr. Rugo presented outcomes after a median follow-up of 5.5 years.
“The two studies were somewhat different in their results…. Where nab-paclitaxel fits into the current treatment armamentarium, I am not sure. The differences by receptor status are interesting but need to be validated.”— C. Kent Osborne, MD, FASCO
Tweet this quote
In the new post hoc analysis, with 4 additional years of follow-up, some differences according to breast cancer subtype have emerged. Nab-paclitaxel showed promising improvements in overall survival and progression-free survival in triple-negative disease, but standard paclitaxel performed better in patients with hormone receptor–positive disease, Dr. Rugo reported.
“In this post hoc subset analysis, there was significant interaction with receptor status between nab-paclitaxel and paclitaxel for progression-free and overall survival,” Dr. Rugo said. “Further investigation is required to explain and validate the subtype specificity seen in this exploration.”
Updated Analysis
ACROSS THE FULL study population, median progression-free survival was similar for the two taxanes: 10.8 months with standard paclitaxel and 9.2 months with nab-paclitaxel (HR = 1.13, P = .16). Median overall survival was also similar: 27.1 months with paclitaxel and 24.2 months with nab-paclitaxel (HR = 1.10, P = .33). The ixabepilone arm, which was closed early due to futility, was inferior across all efficacy measures.
“Our updated results show that progression-free survival for nab-paclitaxel and paclitaxel are still similar, and ixabepilone is still inferior to paclitaxel. At the time of the primary analysis, we saw no difference in overall survival in any of the arms, but in this updated analysis, ixabepilone is inferior to paclitaxel, and with the weekly dosing we used in this trial, nab-paclitaxel still results in overall survival similar to that seen with standard paclitaxel,” she said.
By subset, however, interesting differences have been seen, and the multivariate models for progression-free and overall survival showed significant interactions between treatment and hormone receptor status. In patients with hormone receptor– positive/HER2-negative disease, standard paclitaxel appeared more effective, whereas in triple-negative disease, the opposite was observed (Table 2). Given the limitations of the post hoc assessment, these findings were not powered for statistical significance, Dr. Rugo emphasized.
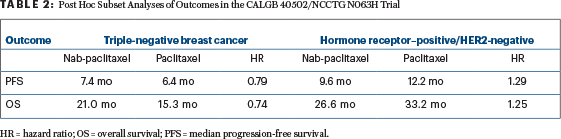
“Numerically, nab-paclitaxel is associated with a 1-month longer progression-free survival, with a hazard ratio of 0.79, but we are not powered to see statistically significant differences in this post hoc analysis,” she said. “Interestingly, in the hormone receptor–positive/HER2-negative subgroup, paclitaxel results in numerically longer progression-free survival (12.2 months) and the longest overall survival (33.2 months)…. Nab-paclitaxel and ixabepilone were clearly inferior to [standard] paclitaxel in this subset.”
By receptor status, in the multivariate model, hazard ratios for the comparison of nab-paclitaxel to paclitaxel were as follows: for hormone receptor– negative patients, 0.71 for progression-free survival (P = .052) and 0.73 for overall survival (P = .078) and for hormone receptor–positive patients, 1.35 for progression-free survival (P = .0047) and 1.30 for overall survival (P = .027).
Grade ≥ 3 adverse events were experienced by 84% in the nab-paclitaxel arm vs 60% in the paclitaxel group. Grade ≥ 3 sensory neuropathy occurred in 27% of those treated with nab-paclitaxel and grade ≥ 3 motor neuropathy occurred in 10%, compared with 18% and 3%, respectively, for standard paclitaxel. Additionally, 26% of
patients discontinued treatment with nab-paclitaxel due to toxicity. For 68% of patients in the nab-paclitaxel arm, the drug was dose-reduced, mostly to 125 mg/m2.
“Adverse events, discontinuations, and dose reductions were more frequent with weekly nab-paclitaxel dosed at 150 mg/m2. This dose should not be used any further in patients with breast cancer,” Dr. Rugo emphasized. She noted that in GeparSepto, a dose of 125 mg/m2 resulted in less toxicity than the higher dose and similar rates of pathologic complete response.
In an interview with The ASCO Post, Dr. Rugo said the study “has clearly shown that weekly paclitaxel is better than every- 3-week paclitaxel [based on historical data], and it continues to be a well-tolerated and efficacious treatment for metastatic breast cancer.”
The other main finding is the “intriguing” benefit seen for nab-paclitaxel in triple-negative breast cancer, especially since it corresponds with GeparSepto data, she commented. “There does appear to be some improvement in efficacy with this drug,” she said. “We showed there may be an advantage to either the dose intensity or the type of chemotherapy in triple-negative breast cancer…. Patients did better with nab-paclitaxel, but it’s hypothesis-generating only.” ■
DISCLOSURE: Drs. Rugo and Osborne reported no conflicts of interest. Dr. Schneeweiss has received honoraria for talks at scientific meetings from Celgene, Roche, Pfizer, AstraZeneca, and Novartis.
REFERENCES
1. Schneeweiss A, Jackisch C, Schmatloch S, et al: Survival analysis of the prospectively randomized phase III GeparSepto trial comparing neoadjuvant chemotherapy with weekly nab-paclitaxel with solvent-based paclitaxel followed by anthracycline/cyclophosphamide for patients with early breast cancer—GBG69. 2017 San Antonio Breast Cancer Symposium. Abstract GS3- 05. Presented December 7, 2017.
2. Untch M, Jackisch C, Schneeweiss A, et al: Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): A randomised, phase 3 trial. Lancet Oncol 17:345-356, 2016.
3. Rugo HS, Barry WT, Moreno-Aspitia A, et al: Long-term follow-up of CALGB 40502/NCCTG N063H (Alliance): A randomized phase III trial of weekly paclitaxel compared to weekly nanoparticle albumin bound nab-paclitaxel or ixabepilone +/- bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer. 2017 San Antonio Breast Cancer Symposium. Abstract GS3-06. Presented December 7, 2017.


