Studies reported at the 2020 Society for Immunotherapy of Cancer (SITC) Annual Meeting, which was held virtually, showed promise for the use of intratumoral injections of novel immunotherapies in treating advanced, resectable melanoma.
In the 3-year interim analysis of a global phase II randomized study, injections with talimogene laherparepvec (T-VEC) before surgery improved multiple clinical outcomes in high-risk patients with stage IIIB–IV M1a resectable disease, according to Reinhard Dummer, MD, of University Hospital of Zurich.1 T-VEC is approved by the U.S. Food and Drug Administration (FDA) for the treatment of metastatic melanoma with injectable lesions.

Reinhard Dummer, MD
“This is the first neoadjuvant trial for an approved oncolytic virus in melanoma and the largest randomized, prospectively controlled neoadjuvant melanoma trial completed to date,” Dr. Dummer said.
Also presented at the 2020 SITC Annual Meeting, Diwakar Davar, MD, of the University of Pittsburgh Medical Center Hillman Cancer Center, reported the final results of a small study evaluating intratumoral CMP-001.2 CMP-001 is a CpGA type-A Toll-like receptor 9 agonist that activates tumor-associated plasmacytoid dendritic cells to produce interferon, which in turn induces antitumor systemic immunity. The product has received Fast Track designation by the FDA.

Diwakar Davar, MD
In both approaches, the intratumoral injections were incorporated into nivolumab-based regimens.
How T-VEC Works
T-VEC is a genetically modified herpes simplex virus-1–based immunotherapy that is injected directly into the tumor, where it selectively replicates in tumor cells, resulting in lysis and release of tumor-derived antigens. Granulocyte macrophage-colony-stimulating factor from T-VEC promotes the maturation of antigen-presenting dendritic cells, which in turn activate T cells. Activated T cells proliferate and migrate to both injected and uninjected tumors for T-cell–mediated tumor cell death, causing a systemic antitumor response.
“T-VEC may be suitable for use in the neoadjuvant setting given these attributes: Its administration requires intralesional injection into tumors, and the mechanism of action invokes systemic immune surveillance,” Dr. Dummer said.
Study of T-VEC
The study enrolled 150 patients with resectable stage IIIB–IV M1a melanoma and at least one injectable lesion. Many patients had in-transit metastases, which would typically exclude them from clinical trials that focus solely on lymph node metastasis, Dr. Dummer said.
Patients were randomly assigned to receive six doses over 12 weeks of neoadjuvant T-VEC followed by surgery or surgical resection. The primary endpoint was relapse-free survival, which was defined as the time from randomization to the first local, regional, or distant recurrence or death; for this analysis, patients who did not receive surgery (mostly in the neoadjuvant group, due to delays) were imputed as events at baseline (ie, disease progression).
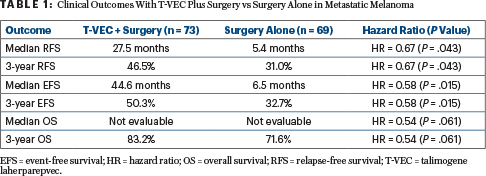
To eliminate confounding, an exploratory relapse-free survival sensitivity analysis censored events at the start of subsequent therapy. Secondary endpoints included overall survival and event-free survival. All P values were descriptive. The percentage of patients who ultimately underwent surgical resection was 93% in the surgery arm and 75% in the T-VEC arm.
Multiple Outcomes Improved With T-VEC
Numerous clinical outcomes were significantly improved with neoadjuvant T-VEC (Table 1). After the Kaplan-Meier curves began to separate, relapse-free survival remained relatively stable over time and is appearing to plateau. The “descriptive statistics” are also now showing “a clear trend that neoadjuvant treatment has a beneficial effect on overall survival,” Dr. Dummer said.
As expected, the surgery cohort was more likely to receive subsequent treatment in the adjuvant/metastatic setting (76.8% vs 50.7%), primarily immunotherapy (46.4% vs 32.9%). With censoring at the time of subsequent therapy, the median event-free survival was 44.6 months with T-VEC vs 5.4 months with surgery alone (hazard ratio = 0.46; P = .002).
“The fact that 14% more surgery patients received subsequent immunotherapy is, I think, a good argument that the effects we see on overall and relapse-free survival are not caused by improved second-line treatments,” he suggested.
Comparison of T-VEC and Checkpoint Inhibitor Monotherapy
When asked how the results with T-VEC stack up to those emerging for checkpoint inhibitors used in the neoadjuvant setting, Dr. Dummer responded: “That’s a difficult question.”
Essentially, compared with nivolumab plus ipilimumab, he said, “the results don’t look very attractive,” but he pointed out this study’s population is particularly “difficult” because of their in-transit metastases, which disqualifies them for combination immunotherapy trials. Also, the fact that adjuvant therapy varies in use, even among patients who received neoadjuvant therapy, further complicates any comparisons, he added.
Dr. Dummer continued: “I would think the combination of ipilimumab and nivolumab should be more powerful than T-VEC in the neoadjuvant setting. However, T-VEC does have an impact and would be a perfect partner in combination with an anti–PD-1 agent.”
The final results of the study will be reported after 5 years of follow-up.
Study of CMP-001
The study evaluating CMP-001 enrolled 30 patients who received 7 weeks of weekly CMP-001 plus 3 doses of nivolumab before surgery. After resection, nivolumab and CMP-001 were dosed every 4 weeks for 48 weeks.
All but one patient completed the full neoadjuvant regimen, with no delays in planned surgery. No dose-limiting toxicities were observed. Radiographic responses were observed in 43% of patients, while 30% achieved stable disease.
Pathologic responses (< 50% residual viable tumor) were observed in 70% (18/30) evaluable patients, of whom 50% (15/30) had a pathologic complete response. Median relapse-free survival in all responders (0%–50% residual viable tumor) was 17 months, but not reached in patients who had a pathologic complete response. Response to neoadjuvant CMP-001/nivolumab was associated with dramatic increases in CD8+ tumor-infiltrating lymphocytes, presence of activated CD8+ T cells peripherally, and the presence of intratumoral plasmacytoid dendritic cells by multiplex IHC.
DISCLOSURE: Dr. Dummer has intermittent, project-focused consulting and/or advisory relationships with Novartis, Merck Sharp & Dhome, Bristol Myers Squibb, Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, MaxiVAX SA, and touchIME. Dr. Davar has received honoraria from Array BioPharma, Immunocore, Instil Bio, Merck, Tesaro, and Vedanta Biosciences; has served as a consultant or advisor to Instil Bio and Merck; has received research funding from Bristol Myers Squibb, CellSight Technologies, Checkmate Pharmaceuticals, Medpacto, Merck, and Tesaro.
REFERENCES
1. Dummer R, Gyorki D, Hyngstrom J, et al: Three-year results of the phase II randomized trial for talimogene laherparepvec (T-VEC) neoadjuvant treatment plus surgery vs surgery in patients with resectable stage IIIB-IV M1a melanoma. 2020 SITC Annual Meeting. Abstract 432.
2. Davar D, Karunamurthy A, Hartman D, et al: Phase II trial of neoadjuvant nivolumab and intratumoral CMP-001 in high-risk resectable melanoma: Final results. 2020 SITC Annual Meeting. Abstract 303.

