
Steven Vogl, MD
The U.S. Food and Drug Administration (FDA) approved 1 year of extended adjuvant neratinib (Nerlynx) after chemotherapy and a year of trastuzumab (Herceptin) for HER2-positive breast cancer this summer on the basis of the ExteNET trial. Many were surprised at the approval, since the evidence of benefit was slim and early, the toxicity severe, and the trial had been shortened and closed short of planned accrual (presumably in despair) by its first sponsor.1 Dr. Miguel -Martin presented an extended analysis of the study out to 5 years at the European Society for Medical Oncology (ESMO) 2017 Congress in Madrid this fall—prompting this editorial.2
Dr. Martin’s 5-year analysis is based on outcomes of the portion of the study population that consented to give further information. He did not present any overall survival information because the protocol forbids this analysis until 10% of the 2,480 participants have died, and this has not yet occurred.
A Brief History of Neratinib and ExteNET
Neratinib is an irreversible inhibitor of HER1, HER2, and HER4 developed by Pfizer. In a two-cohort phase II study, the drug produced a 56% response rate in patients with HER2-positive metastatic disease who had not received prior trastuzumab and a 24% response rate in those who did get prior trastuzumab.3 Nausea, vomiting, and fatigue were common, but diarrhea was nearly universal (93%) and severe in 21%. Diarrhea was worst in the first week of therapy, improved with loperamide, which was recommended prophylactically, and 99% of patients could continue on the drug.4
Should we recommend extended adjuvant neratinib therapy to patients? ‘No!’ is the short answer.— Steven Vogl, MD
Tweet this quote
Neratinib increased the pathologic complete response rate by 23% when substituted for trastuzumab and given with paclitaxel in one arm of the I-SPY 2 trial.5 Pharma enthusiasm for the drug’s neoadjuvant development waned when ALTTO failed to confirm the adjuvant promise of lapatinib (Tykerb) added to trastuzumab based on a clearly improved rate of pathologic complete response in neoALTTO.6
ExteNET was Pfizer’s attempt to show that extended neratinib therapy after a year of trastuzumab would prevent or delay metastatic disease and death.1 When the Breast Cancer International Research Group (BCIRG) 006 trial showed that node-negative HER2-positive patients had a remarkably good prognosis with modern therapy,7 Pfizer acted to limit accrual of lower-risk (node-negative) patients to ExteNET, and also limited accrual to the first year after completion of trastuzumab, since the relapse rate decreased over time. (Initially, entry was allowed up to 2 years after trastuzumab completion.)
In the ExteNET trial, neratinib produced severe diarrhea (grade 3) in 40% of those assigned to it, leading to dose reductions in 26% and treatment discontinuation in 17%. Perhaps as a result, Pfizer stopped accrual after 2,842 patients were enrolled (of a planned 3,850) and shortened follow-up to 2 years. The goal was presumably to limit monetary losses on a drug considered excessively toxic. Pfizer then sold the rights to neratinib—first to Wyeth, then to the biotech start-up Puma—while retaining a small share of any ultimate profits if the drug ever achieved regulatory approval.
Puma, having no drugs on the market, decided to extend follow-up to 5 years in the patients who agreed to this—2,117 did so (1,028 on neratinib). The “reconsented” population had almost identical entry characteristics to the entire population.2 We are not told whether they also had identical lack of compliance with neratinib therapy, but they probably did. One assumes that those not consenting to extension of the study were censored in the analysis at last known follow-up.
The ExteNET Results
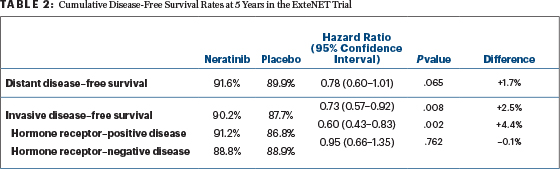
Two-year survival free of invasive disease went from 91.6% for placebo to 93.9% for neratinib (hazard ratio [HR] = 0.67, P = .0091). The initial analysis found most of the benefit in the hormone receptor–positive population (HR = 0.51, P = .0013), with a hazard ratio of 0.93 (P = .74) in the hormone receptor–negative patients (P for interaction = .054).1
The 5-year incidence of recurrences at different locations is given in Table 1. The 5-year invasive disease–free survival and distant disease–free survival are summarized in Table 2. No long-term toxicities were noted from neratinib in the 5-year analysis. The failure to significantly reduce distant metastases noted in Table 2 (distant metastases are the ultimate engine of death from breast cancer) at 5 years in the entire study population suggests that neratinib will not significantly reduce death in the entire population at 10 years, since the median survival after metastases appear is now almost 5 years if pertuzumab (Perjeta) is used as part of initial metastatic therapy.8 Thus, 5-year distant disease–free survival should predict 10-year overall survival.
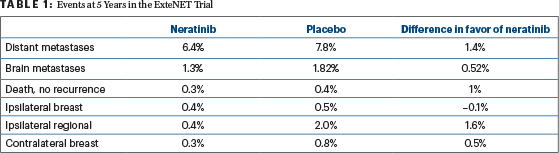
It may turn out that neratinib will more impressively improve overall survival at 10 years in the hormone receptor–positive subset, in which all the prevention of invasive recurrence was observed, especially if the analysis is restricted to those with positive nodes, who seem to have all of the risk.9,10 If all the reduction in distant metastases is in the hormone receptor–positive group, their 5-year distant disease–free survival would be improved by 2.3%. In the estrogen receptor–positive (where all the benefit seems to be in ExteNET), node-positive patients, distant disease–free survival would be improved by 2.9%. If one assumes all the benefit accrues to those who did not stop neratinib forever because of early diarrhea, 5-year distant disease–free survival would be improved by 3.6%. A similar analysis would suggest the 5-year invasive disease–free survival benefit could be as high as 8.3% among those with node-positive, estrogen receptor–positive tumors who did not stop treatment early.
It would be very helpful and reassuring if those analyzing the ExteNET study were to give us this actual subset analysis. If the subset analysis turns out as calculated, this observation, based on restricting the analysis to subsets of subsets, should properly be regarded as hypothesis-generating, and should be confirmed in another trial using a tolerable neratinib dose and schedule.
Implications of Benefit Only for Those With Positive Hormone Receptors
The obvious conclusion is that neratinib is working by some mechanism other than HER2 inhibition, at least in part. It is known to inhibit HER1 (EGFR), HER3, MAPK, MEK, FLT, and FGR.11 It may inhibit “cross-talk” downstream signaling and stimulation of growth via the estrogen receptor. Perhaps adjuvant neratinib only works with concurrent tamoxifen or an aromatase inhibitor, which is how it was given to women with estrogen receptor–positive tumors in ExteNET. One should consider the possibility that HER2 is not its only target; it may be worth exploring its activity against metastatic estrogen receptor–positive, HER2-negative cancers, alone or with conventional endocrine therapy.
Neratinib Toxicity: Explosive Diarrhea
In ExteNET, where no loperamide prophylaxis was mandated or employed, 40% of patients had grade 3 or worse diarrhea (≥ 7 stools per day or requiring hospitalization). An additional 33% had grade 2 diarrhea (4–6 liquid stools per day). This was worst in the first month of therapy (when about three-quarters of the grade 3 diarrhea occurred). There was still a 6% rate of grade 3 diarrhea after the third month, and grade 2 diarrhea had a frequency of 12% in month 12.
Dose reductions for diarrhea occurred in 26% of neratinib patients, and 17% discontinued neratinib for diarrhea at a median of 20 days after entry. There was continued attrition from neratinib therapy during the entire year of planned therapy.
In several small phase II studies sponsored by the neratinib manufacturer (Puma Biotechnology), with a month of prophylactic intensive-dose loperamide starting with the first neratinib dose of 240 mg, grade 3 diarrhea ranged from 10% to 17%.4 Puma thinks this issue is solved4 but has not produced a large trial experience showing good quality of life and compliance data with a year of adjuvant neratinib therapy.
Neratinib Costs a Lot
The cash price of neratinib is about $10,400 for 30 days at full dose. As is now common for oral drugs with a high sticker price, Puma has put in place copay assistance programs to minimize the cost to patients (advertised at $10/mo), while not mitigating the cost to insurance companies or the U.S. Treasury, which ultimately underwrites the Medicare, Medicaid, military, Indian Health Service, and Veterans Affairs prescription programs.
Recommend Extended Adjuvant Neratinib?
Should we recommend extended adjuvant neratinib therapy to patients? “No!” is the short answer. We lack evidence of a substantial benefit for the true endpoint (overall survival) or the best surrogate endpoint (distant disease–free survival) for a tolerable schedule. The 1.7% improvement in distant disease–free survival at 5 years in the entire population is not statistically significant. Further, the monetary cost to society is exorbitant, and the subjective tolerability of a year of neratinib therapy using prophylactic loperamide is not clearly established in a large, defined population.
Should one ever consider extended adjuvant neratinib therapy? In the very high-risk situation of a locally advanced HER2-positive, estrogen receptor–positive breast cancer with many positive nodes and involved margins of resection, with residual disease after neoadjuvant chemoimmunotherapy including pertuzumab, I personally would discuss extended adjuvant neratinib with the patient. ■
DISCLOSURE: Dr. Vogl reported no conflicts of interest.
At Microphone 1 is an occasional column written by Steven Vogl, MD, of the Bronx, New York. When he’s not in his clinic, Dr. Vogl can generally be found at major oncology meetings and often at the microphone, where he stands ready with critical questions for presenters of new data. The opinions expressed in this column reflect those of the author and do not necessarily reflect the opinions of ASCO or The ASCO Post.
REFERENCES
1. Chan A, Delagoe S, Holmes FA, et al: Neratinib after trastuzu-mab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET). Lancet Oncol 17:367-377, 2016.
2. Martin Jimenez M, Holmes FA, Ejlertsen B, et al: Neratinib after trastuzumab-based adjuvant therapy in early-stage HER2+ breast cancer: 5-year analysis of the phase III ExteNET trial. 2017 ESMO Congress. Abstract 149O. Presented September 8, 2017.
3. Burstein HJ, Sun Y, Dirix LY, et al: Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 28:1301-1307, 2010.
4. Ustaris F, Saura C, Di Palma J, et al: Effective management and prevention of neratinib-induced diarrhea. Am J Hematol Oncol 11:13-22, 2015.
5. Park JW, Liu MC, Yee D, et al: Adaptive randomization of neratinib in early breast cancer. N Engl J Med 375:11-22, 2016.
6. Plieth J: How another low-profile trial could swing Puma. EP Vantage. Posted June 11, 2014. Available at epvantage.com/Universal/View.aspx?type=Story&id=513088. Accessed November 10, 2017.
7. Slamon DJ, Eiermann W, Robert NJJ, et al: Ten year follow-up of the BCIRG-006 trial comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC-T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer patients. 2015 San Antonio Breast Cancer Symposium. Abstract S5-04. Presented December 11, 2015.
8. Swain SM, Baselga J, Kim SB, et al: Pertuzumab, trastuzu-mab, and docetaxel in HER2-postive metastatic breast cancer. N Engl J Med 372:724-734, 2015.
9. Tolaney SM, Barry WT, Dang CT, et al: Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372:134-141, 2015.
10. von Minckwitz G, Procter M, de Azambuja E, et al: Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377:122-131, 2017.
11. Rugo H, Chien AJ: HER2-positive breast cancer: Is more treatment better? Lancet Oncol 17:268-270, 2016.

