
Ellin Berman, MD
BOSUTINIB ( BOSULIF) is the latest tyrosine kinase inhibitor that has shown a superior molecular response profile when compared with imatinib.1,2 An orally available dual SRC/ABL1 inhibitor, the drug was shown in preclinical studies to have a potent inhibitory activity against BCR-ABL1 and minimal activity against KIT and platelet-derived growth factor receptor, which has the potential advantage of a more limited nonhematologic toxicity profile.3,4 The tyrosine kinase SRC is a member of the SRC family kinases, which function as key regulators of signal transduction; overexpression of SRC kinases has been found in several malignancies, such as carcinomas of the lung, colon, pancreas, and breast.5,6
Bosutinib at a dose of 500 mg daily is currently licensed for use in chronic myeloid leukemia (CML) as second-line or later therapy for patients who have either progressed on or are intolerant of the first-line therapies imatinib, dasatinib (Sprycel), or nilotinib (Tasigna). Because of the promising results in phase II trials, the manufacturer conducted a randomized trial comparing bosutinib and imatinib in untreated patients with CML (BELA trial).1
BELA Trial Results
THERE WAS NO significant difference in the incidence of complete cytogenetic response between bosutinib and imatinib, 70% vs 68%, which was the primary aim of the trial. However, there was a higher proportion of patients who achieved a major molecular response at 12 months on the bosutinib arm compared with the imatinib arm, 41% vs 27%, a shorter time to response, and a lower rate of transformation to accelerated or blast-phase disease.
Adverse events (any grade) with bosutinib vs imatinib were significantly more common for gastrointestinal events, including diarrhea (70% vs 26%, P < .001), vomiting (33% vs 15%, P < .001); elevated alanine transaminase (ALT; 33% vs 9%, P < .001), and elevated aspartate transaminase (AST; 28% vs 19%, P < .001). Adverse events significantly less common with bosutinib included edema (periorbital 2% vs 14%, P < .001; peripheral 5% vs 12%, P = .006), myalgia (5% vs 12%, P = .010), muscle cramps (5% vs 22%, P < .001), neutropenia (13% vs 30%, P < .001), and leukopenia (9% vs 22%, P < .001). There were no differences in cardiac or vascular events between the two groups.
Although major molecular response was not the primary endpoint of the BELA trial, it is considered to be the more important endpoint, as it represents an approximate 1-log deeper response in terms of BCR-ABL1 transcript level than complete cytogenetic response, and has been shown to predict a longer duration of complete cytogenetic response.7 However, as complete cytogenetic response was chosen as the primary endpoint, and there was no difference between the treatment arms, the trial technically did not meets its primary aim.
BFORE Trial Findings
BECAUSE OF THE 41% incidence of major molecular response in the bosutinib arm, the manufacturer sponsored a second trial, called the BFORE, with the same design, only this time major molecular response was the primary endpoint; the BFORE trial is reviewed in this issue of The ASCO Post. This study used a lower dose of bosutinib, 400 mg daily, but again showed superior results with bosutinib compared with imatinib with respect to major molecular response at 12 months, 47% vs 36%, thereby meeting its primary endpoint.2 In addition, the complete cytogenetic response at 12 months was superior on the bosutinib arm compared with the imatinib arm, 77% vs 66%. The results of the two trials are shown in Table 1.
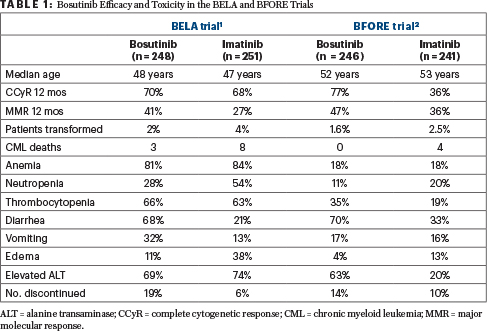
TABLE 1 : Bosutinib Efficacy and Toxicity in the BELA and BFORE Trials
Major Molecular Response: Landmark Laboratory Assessment
ONE VERY IMPORTANT feature noted in both the BELA and BFORE trials was that the time to major molecular response was shorter on the bosutinib arm than on the imatinib arm: in the BELA trial, the median time was 37 weeks on the bosutinib arm compared with 72 weeks on the imatinib arm. The achievement of major molecular response by 12 months is considered a landmark laboratory assessment, as a major molecular response is considered an “optimal” response that has been shown in many studies to correlate with a longer duration of complete cytogenetic response and event-free survival.7,8 The National Comprehensive Cancer Network® (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®), for example, suggest that patients who do not achieve a major molecular response by 12 months be considered for a change in tyrosine kinase inhibitor therapy.9
The rates of major molecular response at 12 months in both the BELA1 and BFORE2 trials (41% and 47%) compare favorably with those seen with nilotinib (44% for the 300-mg dose)10 and dasatinib (46%)11 when imatinib was the comparator drug (22% and 28%, respectively). Importantly, bosutinib was similar to nilotinib and dasatinib in terms of faster achievement of complete cytogenetic response and major molecular response compared with imatinib.
“Bosutinib is a worthy new addition to the list of tyrosine kinase inhibitors effective in the first-line treatment of CML, with a rate of major molecular response at 12 months matching that of dasatinib and nilotinib.”— Ellin Berman, MD
Tweet this quote
The toxicity profile of bosutinib was different from that of imatinib, with gastrointestinal side effects, particularly diarrhea, being more frequent. However, diarrhea was typically of low severity and resolved in most patients with the use of loperamide or diphenoxylate/atropine. Elevated levels of AST and ALT were seen more frequently in the bosutinib arm; in the BFORE trial, the most common cause of bosutinib discontinuation was elevated liver enzymes.2
In both the BELA1 and BFORE2 trials, the incidence of cardiac side effects was similar: 4% with bosutinib vs 3% with imatinib in the BELA trial and 5% vs 5% in the BFORE study. In both trials, QTc prolongation was the most common side effect.
Closing Thoughts
BOSUTINIB IS a worthy new addition to the list of tyrosine kinase inhibitors effective in the first-line treatment of CML, with a rate of major molecular response at 12 months matching that of dasatinib and nilotinib. For many investigators, it was frustrating not to have the drug approved for this indication after the BELA trial, as the major molecular response data were impressive and statistically significant, albeit not the primary endpoint of the study. The leukemia community is pleased the U.S. Food and Drug Administration has approved bosutinib for first-line use; in this instance, tyrosine kinase inhibitor number 3 is definitely not crowding the field.
Dr. Berman is Professor of Medicine, Leukemia Service, Memorial Sloan Kettering Cancer Center, New York.
DISCLOSURE: Dr. Berman reported no conflicts of interest.
REFERENCES
1. Cortes JE, Kim DW, Kantarjian HM, et al: Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Results from the BELA trial. J Clin Oncol 30:3486-3492, 2012.
2. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al: Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: Results from the randomized BFORE trial. J Clin Oncol. November 1, 2017 (early release online).
3. Puttini M, Coluccia AM, Boschelli F, et al: In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant BCR-ABL+ neoplastic cells. Cancer Res 66:11314-11322, 2006.
4. Golas JM, Arndt K, Etienne C, et al: SKI-606, a 4 anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res 63:375-381, 2003.
5. Thomas SM, Brugge JS: Cellular functions regulated by the Src family kinases. Annu Rev Cell Dev Biol 13:513-609, 1997.
6. Summy JM, Gallick GE: Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev 22:337-358, 2003.
7. Iacobucci I, Saglio G, Rosti G, et al: Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res 12:3037-3042, 2006.
8. Marin D, Milojkovic D, Olavarria E, et al: European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood 112:4437-4444, 2008.
10. Saglio G, Kim DW, Issaragrisil S, et al: Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 362:2251-2259, 2010.
11. Kantarjian H, Shah NP, Hochhaus A, et al: Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 362:2260-2270, 2010.


