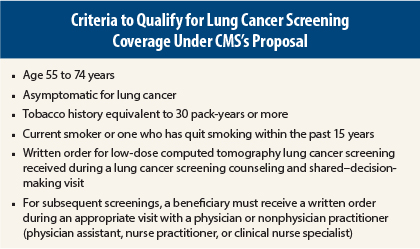
In a long-awaited decision, the Centers for Medicare & Medicaid Services (CMS) has issued a preliminary proposal to cover annual lung cancer screening with low-dose computed tomography for appropriate beneficiaries following counseling and a shared–decision-making visit with a qualified provider. CMS is seeking comments on the proposed decision for a 30-day period and is expected to issue a final decision in February 2015.
Reacting to the CMS announcement, Laurie Fenton Ambrose, President and Chief Executive Officer of Lung Cancer Alliance (LCA), told The ASCO Post, “CMS got it right by acknowledging there is sufficient evidence to add lung cancer screening as a preventive service benefit under the Medicare Program. As a result, we will save tens of thousands of lives as this moves forward. During the comment period we will again reinforce what the American College of Radiology (ACR), Society of Thoracic Surgeons (STS), Lung Cancer Alliance, and ASCO, among others, have put forth for the U.S. Preventive Services Task Force (USPSTF)-recommended population—a pathway forward that will guide responsible, high-quality, and accountable care that does not place barriers to access for patients needing this service nor medical centers providing this benefit.”
Uphill Battle
In April 2014, the Medicare Evidence Development and Coverage Advisory Committee voted against recommending national Medicare coverage for lung cancer screening, igniting intense pushback from more than 40 medical societies. According to data from the Lung Cancer Alliance, approximately 4 million Medicare beneficiaries fit the criteria for screening eligibility. In a press release, the Lung Cancer Alliance pointed out that the new CMS decision is aligned with recommendations previously submitted to CMS by the USPSTF and a coalition that included nearly 100 professional organizations.
Ella A. Kazerooni, MD, Professor of Radiology at the University of Michigan Health System and Chair of the American College of Radiology Lung Cancer Screening Committee, told The ASCO Post, “My overall reaction to the CMS proposal is a very favorable coverage decision for 65- to 74-year-old Medicare beneficiaries, but I’d like to see some clarity about what the path forward will be for those high-risk beneficiaries who are 75 to 80 years old. We recognize that it is highly unlikely that there will ever be a randomized controlled clinical trial conducted in that age population, so I’d like to see what level of evidence is going to be expected to extend coverage to these individuals that the USPSTF recommended screening for.”
Asked about implementing a national lung cancer screening program, Dr. Kazerooni commented, “I think it’s a reach to think that we’ll see an immediate rollout of programs and complete national acceptance of lung cancer screening. It took 20 to 30 years for mammography to reach the levels of adherence we see today, and it will take time to educate providers and the public about the lifesaving value of low-dose computed tomography. However, as opposed to the mammography story, we have electronic health records that can be set up to fire best-practice alerts, based on a patient’s age and smoking history. Electronic media can also be used as an educational tool for shared decision-making, so we have a leg up on the roll out of lung cancer CT screening that should bring it to patients sooner.”
CMS Sets Standardized Criteria
Some experts at the Medicare Evidence Development and Coverage Advisory Committee meeting questioned whether imaging centers across the country could meet the same standards as the participating centers in the landmark National Lung Cancer Screening Trial, which showed a 20% survival benefit in high-risk participants undergoing low-dose computed tomography. Regarding that concern, the CMS proposal set stringent eligibility criteria for participating facilities. Each center must have participated in past lung cancer screening trials or be an accredited advanced diagnostic imaging center with training and experience in low-dose computed tomography lung cancer screening. Centers must also collect and submit data to a CMS-approved national registry for each low-dose computed tomography lung cancer screening performed.
Moreover, participating radiologists must have a current certification with the American Board of Radiology, documented training in diagnostic radiology and radiation safety, and involvement in the supervision and interpretation of at least 300 acquisitions of chest computed tomography in the past 3 years. Organizations like the ACR have been working collaboratively with CMS to set these standards, and through the ACR CT accreditation program with special designation for lung cancer screening centers, and a soon-to-be-released ACR National Radiology Data Registry clinical practice lung cancer screening quality registry to benchmark practices and practitioners, are prepared to meet these requirements.
Affordable Care Act
The Affordable Care Act will require Medicare to reimburse for screening, but beginning in 2015, private insurers must also cover lung cancer screening without cost-sharing. Adding more costs to our exploding health-care system’s budget raises concern among certain health-policy pundits.
To those worried about costs associated with low-dose computed tomography, nationally regarded lung cancer expert James Mulshine, MD, Director, Rush University Translational Sciences Consortium, responded, “The ultimate straw man is cost. But because low-dose computed tomography screening for lung cancer is targeted to a readily definable high-risk population, it can potentially be deployed at a lower expense than other cancer screening tests that are population wide. At various levels, lung cancer screening, especially when appropriately integrated with smoking cessation, may represent one of the best investments of CMS resources in terms of improving outcomes for cancer.”
Dr. Mulshine also addressed another concern of those who have opposed national coverage for low-dose computed tomography: false-positive results. He explained that recent papers have shown that the rate of false-positive results in low-dose computed tomography can actually be lower in lung cancer screening than in many other methods of cancer screening simply by standardizing the thoughtful protocols that are already in use today, such as with the proposed ACR approach.
Dr. Mulshine has been working with the large consortium organized by LCA, ACR, and STS that has engaged CMS to recommend constructive approaches to optimize the screening process now that the coverage decision will soon be enacted. This is a critical time for the lung cancer community to work together on multiple goals that can be leveraged with the CMS proposal.
“First we need to have screening-appropriate tobacco-cessation initiatives to help those who have been addicted to tobacco for 30 or 40 years quit,” he said. “We also have to ensure that our screening delivery systems are accessible and patient-friendly. We have to partner with the surgical societies to make sure that we’re on the glide path to developing less invasive surgical methods. We have to work with the medical oncology community to develop agents directed at early-stage lung cancer. If we stick to these goals, we will see a marked decrease in the incidence and mortality of this devastating disease. However, work still remains, as it would be tragic if overburdensome processes restricted access to this critical new cancer screening service.”
Dr. Mulshine added, “Even with the convincing body of evidence that low-dose computed tomography can save tens of thousands of lives, it’s important to note that if it hadn’t been for the coalition that the LCA assembled with the ACR and the STS along with over 100 partners and even with the critical provisions within the Affordable Care Act, all this work came perilously close to dying on the vine. This has been a remarkable collaborative effort to ensure that this opportunity to turn the tide for the victims of lung cancer is finally realized.” ■
Disclosure: Ms. Fenton Ambrose and Drs. Kazerooni and Mulshine reported no potential conflicts of interest.