In the phase III IMROZ trial, the addition of the anti-CD38 monoclonal antibody isatuximab-irfc to bortezomib, lenalidomide, and dexamethasone (VRd) was more effective than VRd alone as initial therapy in patients ≤ 80 years with newly diagnosed multiple myeloma ineligible for transplant, investigators reported at the 2024 International Myeloma Society Annual Meeting.1

Meletios Dimopoulos, MD
Isatuximab plus VRd reduced the risk of disease progression or death by 40% and improved the achievement of measurable residual disease (MRD) negativity, almost doubling the sustained MRD negativity rate for at least 12 months, reported Meletios Dimopoulos, MD, of National and Kapodistrian University of Athens, Greece. The findings were concurrently published in The New England Journal of Medicine.2
“IMROZ is the first global phase III study of an anti-CD38 monoclonal antibody in combination with VRd in patients with transplant-ineligible newly diagnosed myeloma. The improved efficacy of [isatuximab plus] VRd followed by [isatuximab plus] VRd, in combination with a consistent safety profile, provides an important treatment option for front-line disease control and establishes [isatuximab plus] VRd as a new standard of care” for patients up to age 80 who are not eligible for transplant,” Dr. Dimopoulos said.
Rationale and IMROZ Details
As Dr. Dimopoulos explained, VRd is a standard first-line treatment for newly diagnosed multiple myeloma. Phase III studies have shown improved outcomes with quadruplet regimens that add an anti-CD38 monoclonal antibody to a proteasome inhibitor and immunomodulatory agent in transplant-eligible patients, reinforcing quadruplet therapies as the standard of care. Whether the addition of isatuximab to the VRd regimen would reduce the risk of disease progression or death among transplant-ineligible patients is unclear and was the aim of the IMROZ trial.
KEY POINTS
- The phase III IMROZ trial evaluated the addition of isatuximab to bortezomib, lenalidomide, and dexamethasone (VRd) as compared to VRd alone in patients with newly diagnosed multiple myeloma up to age 80 who were not eligible for transplant.
- Isatuximab/VRd reduced the risk of disease progression or death by 40% (P < .001).
- Isatuximab/VRd essentially doubled the percentage of patients with sustained MRD negativity after 12 months.
- The FDA has approved the combination for this patient subset.
The international phase III trial randomly assigned 446 newly diagnosed patients aged ≤ 80 and not eligible for transplant to isatuximab plus VRd or VRd alone as initial therapy, followed by the same regimen minus bortezomib as maintenance therapy. The median age was 72; more than one-fourth of patients were 75 to 80 years old, and approximately one-fourth had high cytogenetic risk. The primary endpoint was progression-free survival.
Of note, bortezomib at 1.3 mg/m2 was given twice weekly, as mandated by the U.S Food and Drug Administration (FDA) in this registration trial. “We all know that when using this combination in the clinic, most patients will receive weekly bortezomib,” he said.
Isa-VRd Benefits Observed
“The primary endpoint was met (Table 1). The curves separated nicely from the beginning,” Dr. Dimopoulos announced, noting that the control arm also performed well. “We saw a statistically significant 40% reduction in the risk of progression or death. Almost all subsets of patients benefited from the addition of isatuximab, including some difficult-to-treat populations with negative prognostic factors…. And [isatuximab plus] VRd resulted in a notable delay in time to next treatment, consistent with the progression-free survival improvement.”
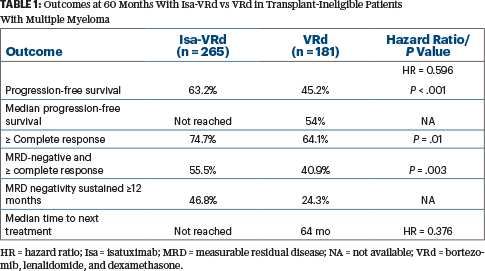
At data cutoff, disease progression after retreatment had not been reached in either arm, but the benefit of isatuximab plus VRd over VRd alone was maintained through the subsequent line of therapy (hazard ratio = 0.697), he noted.
At a median follow-up of 5 years, overall survival is immature, “but it is reassuring to see a trend for a survival advantage [22.4% risk reduction] in patients treated with [isatuximab plus] VRd, despite the fact that crossover was possible for patients [with disease progression] on VRd,” he said. At 60 months, with 128 events occurring, overall survival rates were 72.3% vs 66.3%.
Safety Acceptable
No new safety signals were observed with isatuximab/VRd, and rates of serious adverse events and those leading to treatment discontinuation were similar. “One has to take into account that the exposure of patients to treatment with [isatuximab plus] VRd was much longer [53 months vs 31 months]. For time-adjusted toxicity, there were no significant differences,” Dr. Dimopoulos said.
Grade ≥ 3 treatment-related adverse events occurred in 92% with isatuximab/VRd vs 84% with VRd alone and were serious in 71% and 67%, respectively. The exposure-adjusted event rate per patient-year was approximately 13% per arm. Because of the intensive administration of bortezomib, peripheral neuropathy occurred in more than half the patients in either arm and was grade ≥ 3 in 7% and 6%, respectively.
“Based on this study, the [FDA] approved this combination for the treatment of non–transplant-eligible patients,” he noted.
EXPERT POINT OF VIEW

Thomas G. Martin, MD
Thomas G. Martin, MD, Clinical Professor of Medicine, Adult Leukemia and Bone Marrow Transplantation Program, and Associate Director, Myeloma Program, University of California San Francisco, provided his thoughts on recent trials of quadruplet therapy in transplant-ineligible patients with multiple myeloma.
He noted that the standard induction regimen for transplant-ineligible patients has historically been the triplet of bortezomib, lenalidomide, and dexamethasone (VRd), which yields progression-free survival of 40 to 45 months. More than 5 years ago, the MAIA study3 showed that treatment with the CD38 antibody daratumumab in combination with lenalidomide and dexamethasone led to a marked improvement in progression-free survival over standard lenalidomide/dexamethasone. “The median progression-free survival of 63 months was the highest ever reported in a trial of newly diagnosed transplant-ineligible patients,” he noted.
IMROZ and CEPHEUS Studies
Recently, three large phase III trials have investigated quadruplet-based therapy vs triplets. All three have reported improvements in either progression-free survival or the achievement of measurable residual disease (MRD)-negative complete responses, he said.
The IMROZ study investigated the CD38 monoclonal antibody isatuximab in combination with VRd, comparing this quadruplet with the standard triplet of VRd. There was a progression-free survival rate of 63% at 60 months with isatuximab/VRd, vs 45 months with VRd alone, along with marked improvements in complete response and an MRD-negative complete response. With a projected median progression-free survival of 80 to 90 months, the isatuximab/VRd quadruplet was approved by the U.S. Food and Drug Administration.
In step with IMROZ, the CEPHEUS trial4 compared daratumumab plus VRd with VRd alone and reported very similar results. “In both CEPHEUS and IMROZ, patients were aged 80 or younger with a good performance status. Although several [subsequent] analyses have been performed to look at frailty in the patients included in these trials, it remains unclear if quadruplets can be used in a truly frail population,” Dr. Martin maintained. “That said, there were no new or emerging adverse events that were significantly more challenging in the quadruplet-containing arms.”
BENEFIT Study
The third randomized study, BENEFIT,5 evaluated the quadruplet of isatuximab/VRd vs the triplet of isatuximab plus lenalidomide/dexamethasone (Rd). There were important differences in this trial in that one arm (isatuximab/Rd) did not contain the proteasome inhibitor bortezomib, and in the bortezomib-containing arm, bortezomib was given weekly 3 out of 4 weeks for 12 months and then every other week for an additional 6 months. “The hope was that less frequent dosing of bortezomib would allow for less neuropathy and thus allow a longer treatment duration of bortezomib,” Dr. Martin explained. In most upfront trials, bortezomib has been given twice weekly for approximately 8 to 9 months.
“Although the follow-up is short [23 months] in BENEFIT, there was a significant improvement in the achievement of MRD negativity and complete response with MRD negativity and a lower incidence of grade ≥ 3 peripheral neuropathy,” he noted. “I think these results demonstrate that quadruplet-based therapy with a CD38 antibody plus VRd should be considered standard of care in transplant-ineligible patients.”
DISCLOSURE: Dr. Dimopoulos has received honoraria from Amgen, BeiGene, Bristol Myers Squibb, Janssen, Sanofi, and Takeda. Dr. Martin has consulted for GSK and Pfizer and has received research funding from Sanofi, Johnson & Johnson, Bristol Myers Squibb, and Amgen.
REFERENCES
1. Facon T, Dimopoulos M, Leleu X, et al: Phase 3 study results of isatuximab, bortezomib, lenalidomide, and dexamethasone (Isa-VRd) versus VRd for transplant-ineligible patients with newly diagnosed multiple myeloma (IMROZ). 2024 International Myeloma Society Annual Meeting. Abstract OA-49. Presented September 26, 2024.
2. Facon T, Dimopoulos MA, Leleu XP, et al: Isatuximab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 391:1597-1609, 2024.
3. Usmani SZ, Facon T, Hungria V, et al: Daratumumab SC + bortezomib/lenalidomide/dexamethasone in patients with transplant-ineligible or transplant-deferred newly diagnosed multiple myeloma: results of the phase 3 CEPHEUS study. 2024 International Myeloma Society Annual Meeting. Abstract OA-63. Presented September 27, 2024.
4. Facon T, Kumar SK, Plesner T, et al: Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 22:1582-1596, 2021.
5. Leleu X, Hulin C, Lambert J, et al: Isatuximab plus lenalidomide and dexamethasone with weekly bortezomib versus isatuximab plus lenalidomide and dexamethasone in newly diagnosed transplant ineligible multiple myeloma. The BENEFIT (IFM 2020-05) study. 2024 International Myeloma Society Annual Meeting. Abstract OA-52. Presented September 26, 2024.

