Neoadjuvant therapy for patients with resectable stage III melanoma has recently emerged as a better approach than resection plus adjuvant therapy. At the European Society for Medical Oncology (ESMO) Congress 2024, updates of pivotal neoadjuvant studies demonstrated the long-standing benefit of this approach and validated it as the current standard of care.1,2 The new findings also cement pathologic complete response as the best means of projecting outcomes.

Georgina V. Long, PhD, MBBS
Georgina V. Long, PhD, MBBS, of the Melanoma Institute Australia, University of Sydney Royal North Shore and Mater Hospitals, put the treatment benefit seen with neoadjuvant therapy into context. She noted the remarkable benefits achieved to date with adjuvant anti–PD-1 monotherapy (pembrolizumab and nivolumab), which render half of the patients recurrence-free at 5 years. Now, neoadjuvant administration of checkpoint inhibitors has nudged out using these agents only in the adjuvant setting in resectable macroscopic stage III melanoma, as demonstrated by the randomized phase II SWOG S1801 study (single-agent pembrolizumab followed by adjuvant pembrolizumab)3 and the randomized phase III NADINA study (ipilimumab plus nivolumab with response-adapted adjuvant therapy),4 where risk reductions of 42% and 68%, respectively, were achieved compared with the standard adjuvant anti–PD-1 monotherapy.
NADINA Trial of Ipilimumab Plus Nivolumab
The NADINA trial included 423 patients, randomly assigned 1:1 to either ipilimumab plus nivolumab in the neoadjuvant setting or standard nivolumab in the adjuvant setting after resection. Those in the neoadjuvant arm received two courses of ipilimumab plus nivolumab followed by resection; patients not achieving a major pathologic response received adjuvant nivolumab if BRAF wild-type or dabrafenib plus trametinib if BRAF-mutated. The control arm underwent resection followed by adjuvant nivolumab. Radiotherapy was given if indicated.

Minke Lucas, MD
Minke Lucas, MD, of the Netherlands Cancer Institute in Amsterdam, shared these comments at the ESMO Congress: “Neoadjuvant ipilimumab plus nivolumab is superior to adjuvant nivolumab and should be considered as a new standard of care. At a median follow-up of 15.4 months, the event-free survival benefit for neoadjuvant ipilimumab plus nivolumab remains consistent with the previously published primary analysis,4 and with this second interim analysis, the distant metastasis–free survival was improved as compared with standard-of-care adjuvant PD-1 blockade.”
NADINA investigators previously reported a 12-month event-free survival, the primary endpoint, of 83.7% with neoadjuvant dual checkpoint blockade vs 57.2% with adjuvant nivolumab.4 After 15 months of follow-up, the updated, 18-month event-free survival is now 80.8% in the neoadjuvant arm and 53.9% in the control arm (hazard ratio [HR] = 0.32; P < .001), and distant metastasis–free survival is 85.7% and 62.4%, respectively (HR = 0.37; P < .001). Patients with stage IIIB and stage IIIC disease derived similar benefit.
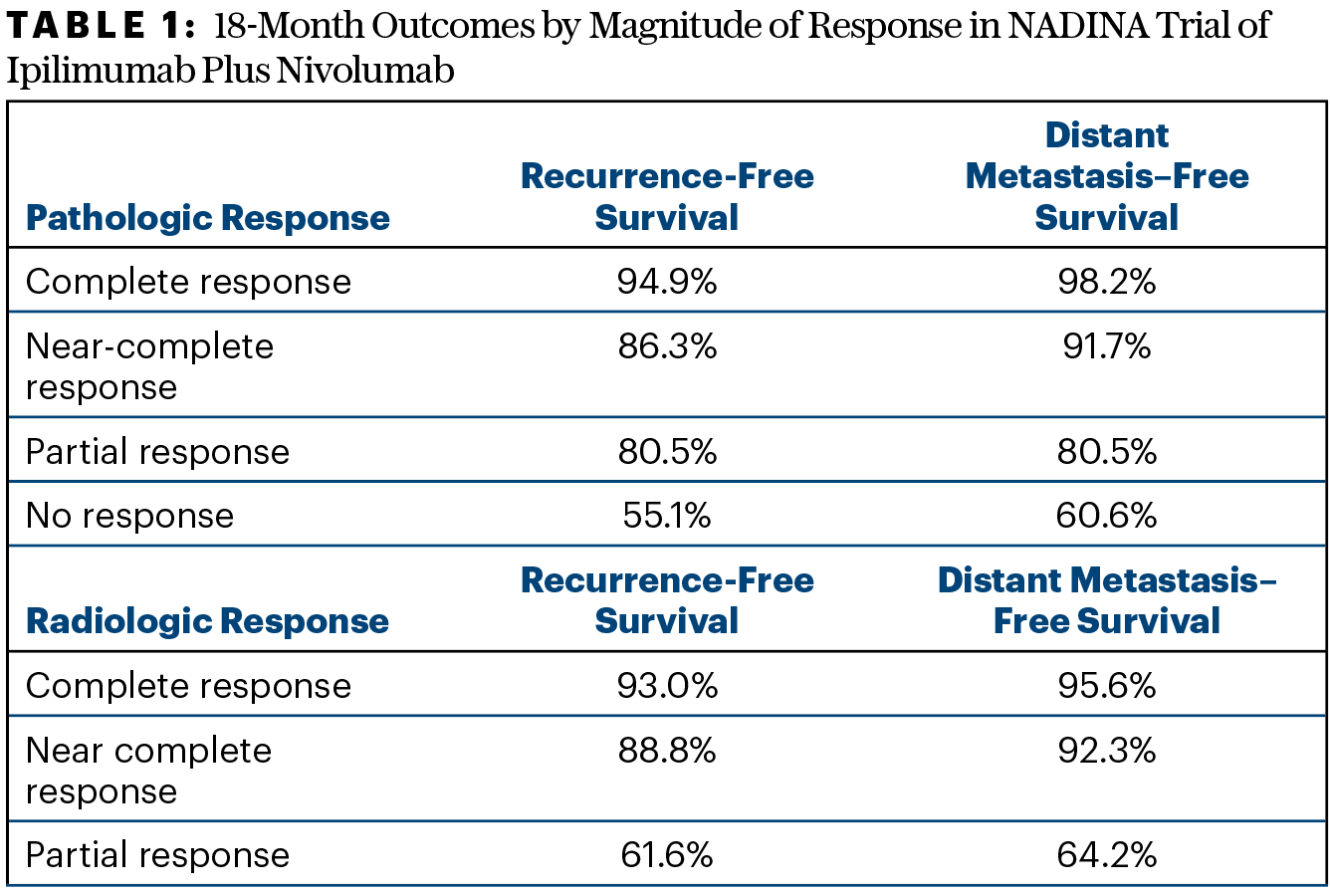
In the final response analysis for the neoadjuvant cohort, 37.2% of patients had a radiologic response, of which 12.7% were complete responses; 68.8% had a pathologic response, of which 60.8% were major and 49.1% were complete. “The majority of patients in the neoadjuvant arm had either a complete pathologic response or a near-complete response. This was different from what we saw for radiologic response, where stable disease was most frequent and not nearly as predictive,” Dr. Long said. The magnitude of response correlated with outcomes at 18 months (Table 1).
Updated Pooled Analysis From INMC
An update from the International Neoadjuvant Melanoma Consortium (INMC), with a median follow-up of 39 months, was presented by Dr. Long. Formed in 2016, the INMC is an international collaboration of melanoma treatment centers around the world. Among 18 melanoma centers in eight countries participating in this analysis, there was a population of 818 patients; 633 were enrolled in clinical trials, and 185 were not. Almost half the patients were treated neoadjuvantly with an anti–PD-1 agent plus an anti–CTLA-4 agent (ie, ipilimumab), whereas most of the others received anti–PD-1 monotherapy; 11% received a BRAF/MEK inhibitor.
Of the 780 patients who underwent surgery, 303 received no adjuvant therapy, and 443 did. There were 193 recurrences altogether. The outcomes were event-free survival, defined from the first dose of neoadjuvant therapy to either disease progression before surgery or recurrence after surgery or death; relapse-free survival, the time from surgery to either recurrence or death; and overall survival from the first dose of drug to death.
Dr. Long reported that the highest rates of major pathologic complete response, recurrence-free survival, and event-free survival were achieved with combination checkpoint inhibition, including major pathologic response rates of 58% to 67% and 3-year event-free survival of 78% overall, regardless of pathologic response.
“No substantial benefit was shown for adding a BRAF/MEK inhibitor to checkpoint inhibitors. And neoadjuvant BRAF/MEK was not as effective as checkpoint inhibition, with no benefit over adjuvant BRAF/MEK,” she said, though acknowledged this is based on cross-trial comparisons. PD-1 inhibitors plus anti–LAG-3 agents (ie, relatlimab) are promising, but data are still scarce, she added.
The best outcomes by far were seen in recipients of anti–PD-1 therapy, with the following 3-year event-free survival data:
- 95% with anti–PD-1 therapy plus any other immunotherapy (n = 31)
- 81% with anti–PD-1 therapy plus anti–LAG-3 therapy (n = 55)
- 77% with anti–PD-1 therapy plus anti–CTLA-4 therapy (n = 351)
- 64% with anti–PD-1 therapy alone (n = 169)
- 37% with BRAF/MEK inhibition (n = 88)
- 78% with any checkpoint inhibitor only combination (n = 437).
Impact of Pathologic Response -to Neoadjuvant Therapy
Similarly, the highest rate of major pathologic response (complete or near-complete pathologic response ie ≤ 10% tumor cells) was reported in patients who received immune checkpoint inhibitors: 46% with anti–PD-1 agents alone, rising to 62% with the addition of anti–CTLA-4 agents in the largest group and to 67% with the addition of anti–LAG-3 agents.
Magnitude of response was an important predictor of recurrence-free survival, as was also shown in NADINA. “Patients with major pathologic responses, regardless of treatment, did better than the other two categories of partial pathologic response or no pathologic response,” Dr. Long explained. In the full cohort of patients who underwent surgery (36 patients did not), 3-year recurrence-free survival approached 90% for pathologic complete or near-complete responders and 68% for partial responders and 40% for nonresponders. The lowest rates, 12% to 15%, were seen for patients with BRAF/MEK inhibitors who were poor responders. Of note, those with a major pathologic response who received neoadjuvant immune checkpoint inhibitors alone had the highest 3-year recurrence-free survival (> 93%) of all therapies administered.
Overall survival at 3 years exceeded 81% regardless of treatment. By pathologic response, more than 97% of complete or near-complete responders were alive at that time, compared with 70% of nonresponders.
Dr. Long concluded: “We need to implement neoadjuvant therapy into routine care, and we need biomarkers to decide who needs anti–PD-1 monotherapy and combination therapy. The neoadjuvant platform is also an excellent and efficient platform for testing new regimens.”
EXPERT POINT OF VIEW

Iván Márquez-Rodas, MD, PhD
The invited discussant of the updates of NADINA and the International Neoadjuvant Melanoma Consortium (INMC) was Iván Márquez-Rodas, MD, PhD, of Spain’s Hospital General Universitario Gregorio Marañón. Dr. Márquez-Rodas focused much of his commentary on questions that remain, despite these studies’ impressive findings.
“After the presentation by Dr. Christian Blank at ASCO 2024 of the NADINA results, there was logical excitement and hype for neoadjuvant therapy. Many voices claimed that ipilimumab/nivolumab should absolutely be the new standard of care. However, even a well-founded translational research clinical trial like NADINA cannot answer all our questions,” he said. The NADINA results also were published in The New England Journal of Medicine.4
The latest analysis of NADINA at approximately 15 months confirms its event-free survival benefit and adds critical information about distant metastasis–free survival, he said. However, “we should be cautious about surrogate endpoints,” as they do not always pan out; overall survival data remain important in establishing a treatment standard.
Major pathologic response correlated with event-free survival in both the NADINA and INMC trials—also with overall survival in the latter—and is a good prognostic marker. “However,” Dr. Márquez-Rodas added, “it is a posttreatment phenomenon” that does not negate the need for pretreatment biomarkers to help tailor treatment.
Finally, there remains the question of the relative superiority of ipilimumab plus nivolumab over single-agent pembrolizumab.3 A head-to-head trial of these therapies would be ideal, he said.
Key Takeaways
Dr. Márquez-Rodas offered these key points based on these clinical trials results:
- The benefit of checkpoint inhibition as neoadjuvant therapy is clear: major pathologic response rates are high and event-free survival is long.
- Targeted therapy has no added value in the neoadjuvant setting.
- Major pathologic response is an “extremely good” prognostic marker.
- Surgery is still important; there are no good predictors of which patients fare well without it.
- Adjuvant therapy is still the desired approach for patients with clinically undetectable stage III melanoma.
- Overall survival data will be important for the generalized adoption of neoadjuvant strategies.
- Although ipilimumab/nivolumab generates higher major pathologic response rates than single-agent pembrolizumab, the two approaches should be formally compared.
DISCLOSURE: Dr. Long has served as a consultant or advisor to Agenus, Amgen, Array Biopharma, AstraZeneca, Bayer, BioNTech, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion, GI Innovation, Hexal AG (Sandoz company), Highlight Therapeutics SL, Immunocore, Innovent Biologics USA, IOBiotech, Iovance Therapeutics, Merck Sharp & Dohme, Novartis, PHMR Ltd, Pierre Fabre, Regeneron, Scancell, and SkylineDX BV. Dr. Lucas reported no conflicts of interest. Dr. Márquez-Rodas reported financial relationships with Amgen, AstraZeneca, BiolineRx, BMS, Celgene, GSK, Highlight Therapeutics, Immunocore, Merck Serono, MSD, Novartis, Pierre Fabre, Regeneron, Roche, Sanofi, and Sun Pharma.
REFERENCES
1. Lucas MW, Menzies AM, Lopez-Yurda M, et al: Distant metastasis-free survival of neoadjuvant nivolumab plus ipilimumab versus adjuvant nivolumab in resectable, macroscopic stage III melanoma: The NADINA trial. ESMO Congress 2024. Abstract LBA42. Presented September 14, 2024.
2. Long GV, Blank CU, Amaria RN, et al: Long-term survival with neoadjuvant therapy in melanoma: Updated pooled analysis from the International Neoadjuvant Melanoma Consortium. ESMO Congress 2024. Abstract LBA41. Presented September 14, 2024.
3. Patel SP, Othus M, Chen Y, et al: Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med 388:813-823, 2023.
4. Blank CU, Lucas MW, Scolyer RA, et al: Neoadjuvant nivolumab and ipilimumab in resectable stage III melanoma. N Engl J Med. June 2, 2024 (early release online).

