In patients with recurrent or metastatic cervical cancer, the addition of the PD-L1–inhibiting monoclonal antibody atezolizumab to first-line chemotherapy plus the angiogenesis inhibitor bevacizumab significantly improved all efficacy outcomes and yielded a median overall survival that exceeded 2.5 years, according to the latest analysis of the phase III BEATcc trial.1

Our results indicate atezolizumab in combination with bevacizumab and platinum-based chemotherapy should be considered a new first-line therapeutic option for patients with metastatic, persistent, or recurrent cervical cancer.— Ana Oaknin, MD, PhD
Tweet this quote
“Our results indicate atezolizumab in combination with bevacizumab and platinum-based chemotherapy should be considered a new first-line therapeutic option for patients with metastatic, persistent, or recurrent cervical cancer,” said Ana Oaknin, MD, PhD, Head of the Gynecologic Cancer Program at Vall d’Hebron Institute of Oncology, Barcelona.
At the November 2023 European Society for Medical Oncology (ESMO) Virtual Plenary, Dr. Oaknin reported the final investigator-assessed progression-free survival and interim overall survival results, which were the study’s dual primary endpoints.1
“The control arm in the BEATcc trial was the GOG 240 regimen,2 which included bevacizumab, so we are building on an existing standard of care rather than just comparing [treatment] with chemotherapy alone,” Dr. Oaknin said. “Benefit from combining atezolizumab with bevacizumab and chemotherapy was achieved without an increase in toxicity, and no new safety signals were observed…. There were similar rates of adverse events, grade ≥ 3 adverse events, adverse events of special interest for bevacizumab, and adverse events leading to any treatment discontinuation.”
As Dr. Oaknin noted, there is a strong rationale for combining immune checkpoint blockade with bevacizumab and chemotherapy. In 2014, GOG 240 demonstrated significantly improved overall survival with bevacizumab added to chemotherapy.2 In 2021, KEYNOTE-826 found that pembrolizumab added to platinum-based chemotherapy—with optional bevacizumab—further improved survival in all comers.3
Both vascular endothelial growth factor and PD-L1 are relevant in cervical cancer pathogenesis, and peripheral immune tolerance and angiogenesis are closely connected in sustaining tumor growth. “Inhibiting both immunosuppression and angiogenesis, therefore, may result in improved and more durable clinical benefit,” she suggested, reporting that the results of BEATcc confirm this hypothesis.
About BEATcc
The open-label randomized BEATcc trial (ENGOT-Cx10/GEICO 68-C/JGOG 1084/GOG-3030) is the first global phase III study to evaluate the addition of atezolizumab to mandatory bevacizumab and platinum-based chemotherapy. The study evaluated the benefit of adding the anti–PD-L1 agent to first-line chemotherapy plus bevacizumab in 401 patients with metastatic (stage IVB), persistent, or recurrent cervical cancer, irrespective of PD-L1 status.
Patients with previously untreated disease not amenable to curative surgery or radiation therapy were randomly assigned to receive standard therapy (cisplatin at 50 mg/m2 or carboplatin AUC 5 plus paclitaxel at 175 mg/m2 plus bevacizumab at 15 mg/kg) with or without atezolizumab at 1,200 mg on day 1 every 3 weeks.
At a median follow-up of 32.9 months, the median treatment duration was 8.5 months in the control arm vs 12.7 months in the atezolizumab arm, with treatment ongoing in 7% vs 23%, respectively. “With a median follow-up of almost 33 months, there are three times as many patients still receiving at least one treatment in the experimental arm compared with the standard arm,” Dr. Oaknin observed.
Both progression-free survival and overall survival were statistically significantly improved with the addition of atezolizumab, with consistent results across both primary and secondary efficacy endpoints (Table 1). Dr. Oaknin commented: “The improvement in subgroups was consistent with the global results favoring atezolizumab, and the similarity between the two Forest plots illustrates the remarkable consistency in treatment effect between the two primary endpoints.”
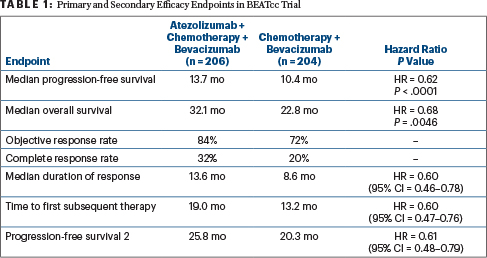
Elaborating on the secondary endpoints, Dr. Oaknin noted the proportion of patients still in response at 3 years was approximately double in the atezolizumab arm, 29% vs 16%. The time to first subsequent therapy and also progression-free survival 2 were improved by almost 6 months as well with atezolizumab on board, with consistent hazard ratios indicating about a 40% reduction in risk.
Grade ≥ 3 adverse events occurred in 75% of the control arm vs 79% of the atezolizumab arm. Safety profiles were as expected with bevacizumab plus platinum-based chemotherapy. Grade 1 or 2 diarrhea, arthralgia, pyrexia, and rash were increased with atezolizumab.
DISCLOSURE: Dr. Oaknin reported financial relationships with AstraZeneca, Clovis Oncology, Deciphera, Genmab, GSK, Immunogen, Mersana Therapeutics, PharmaMar, MSD de Espana SA, Agenus, Sutro, Corcept Therapeutics, EMD Serono, Novocure, Shattuck Labs, iTeos, Eisai, and Roche.
REFERENCES
1. Oaknin A, Gladieff L, Martinez-Garcia J, et al: Primary results from BEATcc (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030), a randomised phase III trial of first-line atezolizumab combined with a platinum doublet and bevacizumab for metastatic (stage IVB), persistent or recurrent cervical cancer. ESMO Virtual Plenary. Abstract VP5-2023. Presented November 3, 2023.
2. Tewari KS, Sill MW, Long HJ 3rd, et al: Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370:734-743, 2014.
3. Monk BJ, Colombo N, Tewari KS, et al: First-line pembrolizumab + chemotherapy versus placebo + chemotherapy for persistent, recurrent, or metastatic cervical cancer. J Clin Oncol. November 1, 2023 (early release online).

