In patients with relapsed or refractory multiple myeloma, the addition of daratumumab to carfilzomib plus dexamethasone improved multiple outcomes, compared with carfilzomib/dexamethasone alone, in the international phase III CANDOR trial.1
“Overall, carfilzomib/dexamethasone/daratumumab was associated with a favorable benefit-risk profile and represents an efficacious new regimen for relapsed or refractory multiple myeloma, including lenalidomide-exposed and lenalidomide-refractory patients,” Saad Z. Usmani, MD, Chief of Plasma Cell Disorders at Levine Cancer Institute, Atrium Health, in Charlotte, North Carolina, reported at the late-breaking abstract session of the 2019 American Society of Hematology (ASH) Annual Meeting & Exposition.

Overall, carfilzomib/dexamethasone/ daratumumab was associated with a favorable benefit-risk profile and represents an efficacious new regimen for relapsed or refractory multiple myeloma….— Saad Z. Usmani, MD
Tweet this quote
In patients with relapsed or refractory multiple myeloma, the proteasome inhibitor carfilzomib and the anti-CD38 monoclonal antibody daratumumab are approved as single agents or as components of combination regimens. The triplet of carfilzomib, dexamethasone, and daratumumab (KdD) was shown to be efficacious and safe in patients with relapsed or refractory myeloma in the phase I MMY1001 study.2 This led to the phase III CANDOR trial, which compared KdD with carfilzomib and dexamethasone (Kd) alone in 466 patients with relapsed or refractory disease.
Dr. Usmani presented the primary analysis of CANDOR, which enrolled 466 patients with up to three prior lines of therapy and who achieved at least a partial response to at least one of those treatments. Of the 466 patients (median age = 64 years), 42.3% received prior lenalidomide-based regimens and 90.3% received previous bortezomib. One-third of patients were refractory to lenalidomide.
Patients were randomly assigned 2:1 to KdD or Kd. All participants received carfilzomib as a 30-minute infusion on days 1, 2, 8, 9, 15, and 16 of each 28-day cycle. Daratumumab (8 mg/kg) was administered intravenously on days 1 and 2 of cycle 1, at 16 mg/kg once weekly for the remaining doses of the first two cycles, then every 2 weeks for four cycles (cycles 3–6), and every 4 weeks thereafter. All patients received 40 mg of dexamethasone (20 mg if aged > 75). The primary endpoint was progression-free survival.
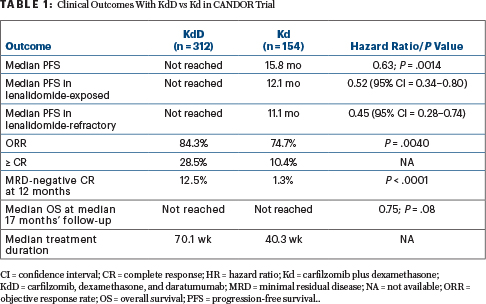
Multiple Endpoints Favored KdD
After a median follow-up of approximately 16 months, -CANDOR met its primary endpoint. Median progression-free survival was not reached for the KdD arm and was 15.8 months for the Kd arm (hazard ratio [HR] = 0.63; P =.0014).
“KdD resulted in a significant progression-free survival benefit over Kd, with a 37% reduction in the risk of progression or death. The progression-free survival benefit of KdD was maintained across prespecified clinically important subgroups, particularly among lenalidomide-exposed and lenalidomide-refractory patients. Patients treated with KdD achieved deeper responses, with a nearly 10-times higher [minimal residual disease]-negative complete response rate than Kd-treated patients,” Dr. Usmani noted. Moreover, multiple parameters favored KdD vs Kd, as shown in Table 1.
The incidence of grade≥ 3 adverse events was 82.1% and 73.9% in the KdD and Kd arms, respectively, with serious adverse events seen in 56.2% and 45.8%. The rate of treatment discontinuations was similar in both arms—around 24%.
The frequency of grade ≥ 3 cardiac failure was 3.9% with KdD and 8.5% with Kd; this led to discontinuation of carfilzomib in 3.9% and 4.6%, respectively. Five deaths were reported as treatment-related, all in the KdD arm (pneumonia, sepsis, septic shock, Acinetobacter infection, and cardiorespiratory arrest [1 each]). ■
DISCLOSURE: Dr. Usmani reported financial relationships with AbbVie, SkylineDx, GSK, Seattle Genetics, Mundipharma, Takeda, Sanofi, Pharmacyclics, Merck, Janssen, Celgene, BMS, Array Biopharma, and Amgen.
REFERENCES
1. Usmani SZ, Quach H, Mateos M-V, et al: Carfilzomib, dexamethasone, and daratumumab vs carfilzomib and dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma: Primary analysis results from the randomized, open-label, phase III study CANDOR (NCT03158688). 2019 ASH Annual Meeting & Exposition. Abstract LBA-6. Presented December 10, 2019.
2. Chari A, Martinez-Lopez J, Mateos MV, et al: Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 134:421-431, 2019.


