Geriatrics for the Oncologist is guest edited by Stuart Lichtman, MD, FACP, FASCO, and developed in collaboration with the International Society of Geriatric Oncology (SIOG). Visit SIOG.org for more on geriatric oncology.
Increasing age is directly associated with an increasing risk of cancer, and persons over age 65 constitute the fastest-growing group in the United States. Not only do older adults comprise the majority of cancer patients, at the same time, they have also been vastly underrepresented in clinical trials. As a result, little evidence-based data exist to guide their course of treatment.
Alternative trial designs and expanded research evaluations are needed to guide cancer therapy in this population, which is estimated to account for 20% of all Americans by the year 2030. There have been proposals to correct the widespread underreporting and underrepresentation of older adults in cancer trials and to reduce the existing barriers to trial enrollment. There are also specific issues of treatment and survivorship as they pertain to older adults, including function, clinical benefit, quality of life, polypharmacy, toxicity, and comorbidity.
Largest Consumers of Chemotherapy
Older cancer patients have traditionally not been the subject of clinical drug development. However, they are the largest consumers of chemotherapy, and their numbers are rising dramatically. Worldwide, 1 of every 10 persons is 60 years old or over. By 2050, one of every five persons will be 60 or older, and by 2150, this ratio will decrease to one of every three persons. By 2050, the actual number of people over the age of 60 will be almost 2 billion, at which point the population of older persons will outnumber children up to 14 years old.
Furthermore, the older population itself is aging. The United Nations Statistics on Population Aging in 2000 noted that the average life span increased by 26 years since 1950, and the proportion of people over 65 years old increased from 1 in 30 to about 1 in 6. Currently, the oldest old—aged 80 years and older—make up 12% of the population over 60, and this segment is the fastest growing of the older population. By 2050, 21% of the older population is expected to be 80 years or older.
Underrepresented in Trials
Increasing age is directly associated with increasing rates of cancer, corresponding to an 11-fold greater incidence in persons over the age of 65 years compared with those under age 65. Consequently, the older population comprises a majority of cancer patients.
Despite the increasing incidence of cancer associated with aging and the aging of the U.S. population, only a relatively small number of older patients have been included in clinical trials. As a result, there is a lack of high-quality data on which to base meaningful decisions. Older adults have been underserved in the area of drug development, and they often go untreated, even when data exist for efficacious therapy.1 The needs of the older cancer patient are simply not being met.
A number of barriers limit the participation of older patients in clinical trials (Table 1). For various reasons, clinicians often do not offer a clinical trial to eligible older patients. Cognitive dysfunction also interferes with patient understanding of complicated informed consent documents; such impairments affect as many as 36% of adults aged 85 and older, thus ruling out trial enrollment.
Need for Novel Trial Designs
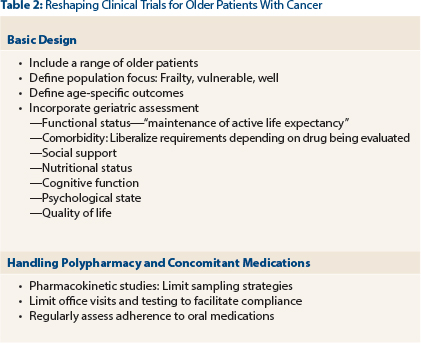
In addition, a number of clinical trial design issues need to be addressed. Specific issues that are particularly pertinent to older patients include function, comorbidity, and social supports. Clinical investigators and biostatisticians must develop novel trials to optimize the data derived from these studies (Table 2). Suggestions have been proposed to “geriatricize” the standard oncology drug design.2 For instance, we have to meld the standard oncology outcomes of disease-specific and overall survival with geriatric outcomes of evaluating function, cognition, toxicity, nutrition, and dependence (ie, quality vs quantity of life).
ASCO has published a position paper on the specific needs to enhance the evidence base to improve cancer care of older patients.3 To meet the needs of these vulnerable cancer patients, which will be the majority, these clinical design considerations should be incorporated in studies as soon as possible. Reluctance to alter our standard designs must be overcome, or the progress that is urgently needed will never occur.4-6 ■
Disclosure: Dr. Lichtman reported no potential conflicts of interest.
References
1. Hurria A, Dale W, Mooney M, et al: Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 32:2587-2594, 2014.
2. Wildiers H, Mauer M, Pallis A, et al: End points and trial design in geriatric oncology research: A joint European Organisation for Research and Treatment of Cancer—Alliance for Clinical Trials in Oncology—International Society of Geriatric Oncology position article. J Clin Oncol 31:3711-3718, 2013.
3. Lichtman SM: Clinical trial design in older adults with cancer—The need for new paradigms. J Geriatr Oncol 3:368-375, 2012.
4. Lichtman SM: Call for changes in clinical trial reporting of older patients with cancer. J Clin Oncol 30:893-894, 2012.
5. Kemeny MM, Peterson BL, Kornblith AB, et al: Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 21:2268-2275, 2003.
6. Hurria A, Levit LA, Dale W, et al: Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology Statement. J Clin Oncol 33:3826-3833, 2015.
GUEST EDITOR
Dr. Lichtman is an Attending Physician at Memorial Sloan Kettering Cancer Center, Commack, New York, and Professor of Medicine, Weill Cornell Medical College, New York. He is also President Elect of the International Society of Geriatric Oncology (www.siog.org).