In the first-line treatment of metastatic pancreatic cancer, the sequencing of nab-paclitaxel plus gemcitabine with FOLFOX (fluorouracil, leucovorin, oxaliplatin) resulted in a significant improvement in overall survival at 12 months and in all other efficacy endpoints as compared with the standard approach in the randomized phase II SEQUENCE trial, which was conducted by the Spanish Cooperative Group for the Treatment of Digestive Tumors.1
The upfront administration of nab-paclitaxel/gemcitabine followed by modified FOLFOX—alternating throughout treatment—resulted in improvements in 12-month overall survival, objective response rate, time to disease progression, progression-free survival, and median overall survival when compared with standard nab-paclitaxel/gemcitabine, Alfredo Carrato, MD, PhD, of Alcalá University and Ramón y Cajal University Hospital, Madrid, Spain, reported at the 2022 ASCO Annual Meeting. Although the sequencing approach involved additional chemotherapy, the safety profile was manageable, and quality of life was not compromised, he added.

Alfredo Carrato, MD, PhD
“A significant improvement in efficacy was observed with nab-paclitaxel/gemcitabine and modified FOLFOX—also when compared with the results of other recently performed trials.2 This regimen represents a step forward and a promising alternative for the benefit of our patients,” Dr. Carrato commented.
“We all want to increase the efficacy of the treatments available for patients with metastatic pancreatic cancer. We know that the basal cell pancreatic adenocarcinoma subtype responds better to nab-paclitaxel plus gemcitabine and has a worse prognosis, and the classic subtype responds better to FOLFIRINOX [fluorouracil, leucovorin, irinotecan, oxaliplatin] and has a better prognosis. However, since it’s not feasible to give these regimens together because of toxicity, we designed a sequential regimen that gives the nab-paclitaxel/gemcitabine followed by FOLFOX instead of FOLFIRINOX. Upfront nab-paclitaxel depletes the stroma and facilitates access of all the drugs to the tumor,” he explained.
About the SEQUENCE Trial
The study randomly assigned 157 patients who had not been treated for metastatic pancreatic adenocarcinoma. More than half the population had at least three metastatic sites. Standard treatment with nab-paclitaxel plus gemcitabine was given on days 1, 8, and 15 every 4 weeks until disease progression. Nab-paclitaxel/gemcitabine was sequenced on days 1, 8, and 15, with modified FOLFOX on day 29, every 6 weeks until disease progression. The primary endpoint was increased efficacy in terms of 12-month overall survival.
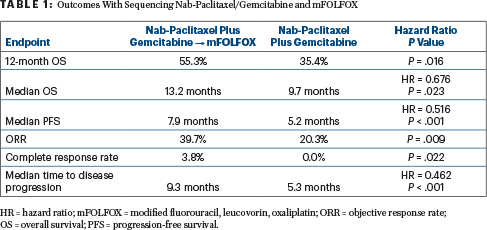
Multiple Endpoints Improved
At 12 months, overall survival rates were 55.3% in the sequencing arm and 35.4% with standard nab-paclitaxel/gemcitabine (P = .016). Median overall survival was 13.2 months vs 9.7 months (hazard ratio [HR] = 0.676; P = .023). The median survival for the control arm was longer than was observed in the landmark MPACT trial (8.5 months), which established nab-paclitaxel/gemcitabine as the standard of care,2 he noted.
“So, the primary endpoint was met,” Dr. -Carrato said. The sequencing approach resulted in improvements in multiple efficacy outcomes (Table 1).
The time on treatment was significantly -longer in the experimental arm (8.3 months vs 4.0 months; P < .001), but this arm also had significantly more dose delays (64% vs 43%; P = .010), dose reductions (71% vs 52%; P = .021), and transient interruptions (82% vs 65%; P = .019). More patients on the control arm received subsequent treatment (55% vs 40%; P = .08), he added.
Safety and Quality of Life
Safety was comparable between the arms except, on the sequencing arm, more grade ≥ 3 neutropenia (46% vs 24%; P = .004) and grade ≥ 3 thrombocytopenia (24% vs 8%; P = .007). Two patients (2.6%) on this arm died due to adverse events (cellulitis and sepsis).
Investigators performed quality-of-life assessments at baseline and at 12, 24, 36, and 48 weeks. The EORTC QLQ-C30 instruments revealed no meaningful differences in physical, emotional, or social functioning; fatigue; nausea, vomiting, constipation, and diarrhea; pain; dyspnea; insomnia; or financial difficulties.
KEY POINTS
- The phase II SEQUENCE trial in patients with metastatic pancreatic cancer evaluated a sequencing approach that alternated nab-paclitaxel/gemcitabine with modified FOLFOX until disease progression.
- The sequencing approach resulted in a significant improvement in 12-month overall survival—55.3% vs 35.4%—with standard nab-paclitaxel/gemcitabine (P = .016).
- Multiple other endpoints were also significantly improved with the sequencing approach.
“We can globally consider that the EORTC QLQ-C30 quality-of-life score was similar in both arms,” Dr. Carrato said, noting these analyses are ongoing.
DISCLOSURE: Dr. Carrato reported financial relationships with Mylan-Viatris, Baxter, MSD, Hutchison MediPharma, and Bristol Myers Squibb.
REFERENCES
1. Carrato A, Pazo-Cid R, Macarulla T, et al: Sequential nab-paclitaxel/gemcitabine followed by modified FOLFOX for first-line metastatic pancreatic cancer: The SEQUENCE trial. 2022 ASCO Annual Meeting. Abstract 4022. Presented June 5, 2022.
2. Von Hoff DD, Ervin T, Arena FP, et al: Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 369:1691-7103, 2013.


